Biomedical Engineering Reference
In-Depth Information
8.2.1 Fluorescent Particles
Fluorescence is one of the major tools used in biochemistry/biotechnology. The high
sensitivity of the technique and the numerous available coupling strategies make it
a very versatile routine tool. Before describing the probes used in this context, let us
first recall a few notions of the physical principle
8.2.1.1 Fluorescence
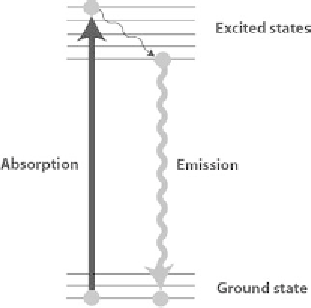
Some molecules have the ability to absorb photons at a given wavelength and emit
them back at a different (longer) wavelength corresponding to a lower energy (Fig-
ure 8.6). Fluorescein for instance is a very popular fluorescent dye that shines in
yellow-green (
λ
~ 520 nm) when excited by blue light (
λ
~ 480 nm). For a given
fluorophore, there is a range of wavelengths that are absorbed (the excitation spec-
trum) and a range of emitted wavelengths (emission spectrum) (Figure 8.7).
This property is used extensively in fluorescence microscopy but also with other
techniques such as flow cytometry (see Section 8.3.3.2). The reasons for such an
extensive use are:
The extreme sensitivity of fluorescence up to a single fluorophore detection.
the huge panel of possible reactants that can be chemically synthetized. With
the right coupling chemistry it is possible to couple a dye to virtually any
biological object of interest.
The advent of naturally fluorescent proteins such as GFP that can be synthe-
sized by transfected or modified cells lines.
·
·
·
Immunofluorescence is a particular coupling strategy that uses biochemistry
rather than chemistry. Antibodies are first covalently coupled to a fluorophore and
then allowed to interact with the cell or the tissue so that only the protein of interest
“becomes” fluorescent.
Figure 8.6
Jablonski energy diagram illustrating the principle of fluorescence.