Biomedical Engineering Reference
In-Depth Information
7.4.2.2 Diffusion Limited Reaction
Introduction
In a biochip, heterogeneous reaction kinetics depends not only on the reaction
rate—as we have seen in the first section of this chapter—but also on the diffu-
sion of species towards the surface where reaction occurs. If diffusion is fast, for
example in the case of very small diffusing molecules, the flux of these reactant
molecules at the wall is such that there is no delay in the reaction. On the other
hand, if diffusion process is slow, there will be depletion near the reacting surface
and the biochemical reaction will be slowed down. In the first case, the process is
limited by the reaction rate, in the second case it is limited by the diffusional flux.
This problem is the subject of an abundant literature [22-26].
In the preceding section, the concentration was supposed uniform (but time-
dependant) in the microchamber volume. In this section, we treat the case where a
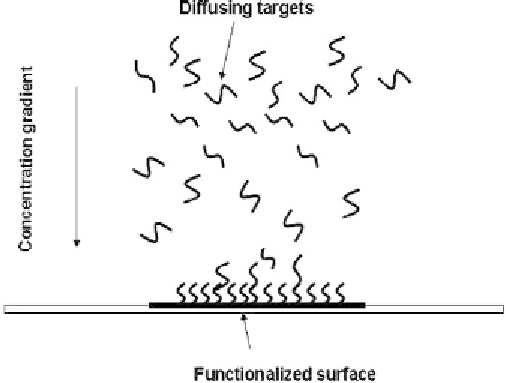
depletion layer is observed near the functionalized surface (Figure 7.36).
Governing Equations
Concentration in the fluid volume is obtained through the usual diffusion equation
∂
∂
c
=
D c
D
(7.74)
t
where
c
is the concentration of substrate,
D
its diffusion coefficient. In a two-
dimensional Cartesian system, this equation can be rewritten as
2
2
æ
ö
∂
c
∂
c
∂
c
(7.75)
=
D
+
ç
÷
2
2
∂
t
è
∂
x
∂
y
ø
Figure 7.36
Schematic view of the diffusing targets and hybridization on the functionalized surface.