Biomedical Engineering Reference
In-Depth Information
This last equation can be rewritten under the form
d
G
=
k c
G -
(
k c
+
k
)
G
(7.37)
0
0
0
on
on
off
dt
Equation (7.37) can be integrated and we obtain
G
k c
on
0
é
-
(
k c
+
k
)
t
ù
=
1
-
e
on
0
off
(7.38)
ë
û
G
k c
+
k
0
on
0
off
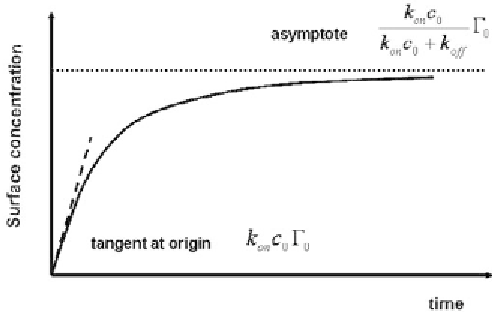
Using (7.38), we obtain the surface concentration kinetics shown in Figure 7.23.
At small times, the exponential term in (7.38) can be developed in a Taylor
expansion and the surface concentration kinetics is the linear function of the time
defined by
G =
o
k c
0 0
t
(7.39)
Equation (7.39) indicates that the kinetics described by the Langmuir equation
(7.36) is rapid if the term
k
on
c
0
is large (i.e., when the adsorption constant on the
surface and the concentration in molecules are large). For longer times, the surface
concentration approaches an asymptotic value defined by
G
k c
k c
¥
on
0
=
(7.40)
G
+
k
0
on
0
off
It can be verified in (7.38) that in the case where
k
off
is zero, the asymptotic value is
then G
0
and the surface is becomes totally saturated. The larger the coefficient
k
off
,
the smaller the value of G
¥
/G
0
.
7.3.3.2 Adsorption and Desorption
Suppose that after the hybridization has reached its asymptotic value, the remaining
targets or analytes in solution are suddenly washed out. Desorption is then the driv-
ing mechanism and the corresponding kinetics is schematized by Figure 7.24.
Figure 7.23
Kinetics of surface concentration from equation (7.38).