Biomedical Engineering Reference
In-Depth Information
Second, the magnitude of
k
for a given reaction order may be very different;
the reason is that for a chemical reaction to proceed, the concerned molecules must
have a closely defined state (for example, orientation). This condition is related to a
probabilistic behavior which in term depends on the activation energy - sometimes
called “Arrhenius” factor. Because the range of activation energy is large, the rate
constants take very different values.
7.3.1.4 Temperature Dependence of the Reaction Constant
A closer look at the “Arrhenius factor” is obtained by considering the dependency
of
k
on the temperature
T
. This property is often used to modify the equilibrium
state of biochemical reactions like DNA hybridization, as we will see later on. The
dependency of the rate constant
k
on the temperature
T
is given by Arrhenius law
E
ln
k
=
ln
A
-
(7.9)
RT
The two parameters
A
and
E
a
/R
are called the Arrhenius parameters; more
specifically,
A
is the “frequency” factor and
E
a
/R
is the activation energy. These
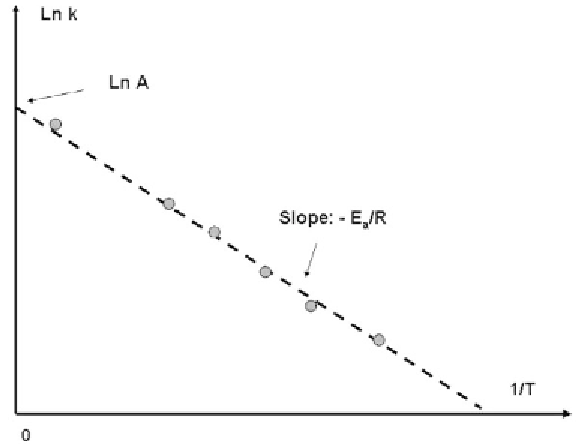
parameters are usually determined graphically from experimental results as shown
in Figure 7.13. The intercept is
ln A
and the value of the slope -
E
a
/RT
.
High activation energy corresponds to a very steep slope and a very important
dependency of
k
on the temperature. For the rate constant
k
be really a constant,
the activation energy must be zero. Another form of (7.9) is
E
RT
-
(7.10)
k A e
=
Figure 7.13
Schematic drawing of the relation (7.9) showing the Arrhenius parameters A and
E
a
/
RT
.