Biomedical Engineering Reference
In-Depth Information
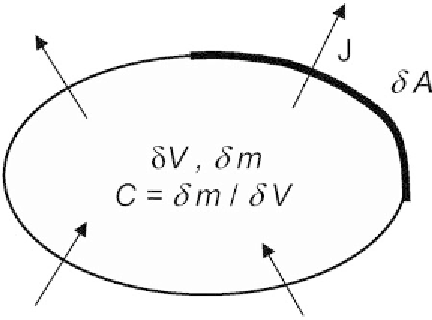
Figure 5.3
Schematization of an elementary volume with a concentration
c
of diffusing
substance.
J
traverses the elementary surface
dA
in a time interval
dt
. The
international unit for concentration is the kilo per cubic meter (kg/m
3
). However,
biologists and chemists often express a concentration in mole/
m
L. In this case, the
concentration is defined by the number of moles inside an elementary volume. The
SI unit for mass flux is kg/m
2
is and we will use a more adapted unit (i.e., mole/
m
m
2
is There is a fundamental law that links the mass flux to the concentration
gradient called Fick's law.
where the mass flux
5.3.1 Fick's Law
Fick's law can be expressed as
�
J
= - Ñ
D c
(5.3)
where
D
is the diffusion constant or coefficient. The SI unit of
D
is m
2
/s, the same
as for the cinematic viscosity n or the thermal diffusivity
a
. All of these quantities
are coefficients in a transport equation either of concentration, velocity, or enthalpy
and characterized by a flux.
5.3.2 Concentration Equation
5.3.2.1 Differential Diffusion Equation
Using Fick's law (5.3) and evaluating the mass balance of a substance in an elemen-
tary volume of carrier liquid, yields the diffusion equation
¶
=
c
div D c
Ñ +
S
(5.4)
¶
(
)
t
In (5.4), the terms
S
stands for a source or sink term of concentration. For
example, if there is a biochemical in some part of the domain, the concentration
of substance may locally appear or disappear. We will come back to this point in
Chapter 6.