Biomedical Engineering Reference
In-Depth Information
even for mercury. In the following sections we analyze successively the characteris-
tics of drops having, respectively, large and small Bond numbers.
Case 1: Large Droplet, Bo >> 1
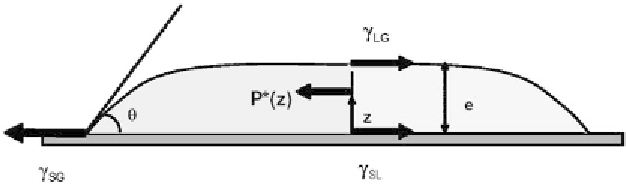
According top the observation of the preceding section, a large droplet has a flat
upper surface and its shape is shown in Figure 3.47.
Let us calculate the height of such a droplet as a function of contact angle and
surface tension. Take the control volume shown in Figure 3.47 and write the bal-
ance of the forces that act on this volume. The surface tension contribution is
S
γ
=
-
γ γ
+
(
(3.67)
)
SG
SL
LG
and the hydrostatic pressure contribution is
e
1
2
(
)
*
ò
2
P
=
ρ
g e
-
z dz
=
ρ
g e
(3.68)
0
The equilibrium condition yields
P
*
+
S
= 0, which results in the relation
1
2
ρ
g e
+
γ
-
γ γ
+
=
0
(
)
(3.69)
SG
SL
LG
2
Recall that Young's law imposes a relation between the surface tensions
γ γ γ
-
=
cos
θ
(3.70)
SG
SL
LG
Upon substitution of (3.70) in (3.69), we obtain
1
2
γ
(
1 cos
-
θ ρ
)
=
g e
LG
2
Using the trigonometric expression 1 - cos
q
= 2sin
2
(
q
/2), we finally find
γ
θ
θ
LG
e
=
2
sin
= �
2 sin
(3.71)
ρ
g
2
2
Relation (3.71) shows that the height of a large droplet is proportional to the capil-
lary length. With the capillary length being of the order of 2 mm, the height of large
droplets is less than 4 mm.
Figure 3.47
Equilibrium of the forces (per unit length) on a control volume of the drop.