Biomedical Engineering Reference
In-Depth Information
Figure 3.45
Comparison of the shape between microdrops and macrodrops (not to scale). Micro-
drops have the shape of spherical caps, whereas larger drops are flattened by the action of gravity
and their height is related to the capillary length.
This observation is linked to the balance between gravity and surface tension.
A microscopic drop is governed solely by surface tension, whereas the shape of a
larger droplet results from a balance between the two forces. The scale length of
this transition
,
is the capillary length (see Chapter 1). We recall that this length is
defined by the ratio of the Laplace pressure to the hydrostatic pressure. If we com-
pare the two pressures for a drop, we obtain
γ
D
P
Laplace
�
»
(3.64)
D
P
ρ
g
�
hydrostatic
where
g
is the surface tension,
ρ
is the density, and
g
is the gravitational constant.
The two pressures are of the same order when
γ
ρ
�
»
(3.65)
g
,
is called the capillary length. A drop of dimension smaller than the capillary
length has a shape resembling that of a spherical cap. A drop larger than the capil-
lary length is flattened by gravity. Note that a dimensionless number—the Bond
number—can be derived from (3.64) yielding a similar meaning. The Bond number
is expressed by
2
Bo
ρ
gR
=
(3.66)
γ
where
R
is of the order of the drop radius. If
Bo
< 1, the drop is spherical, or else the
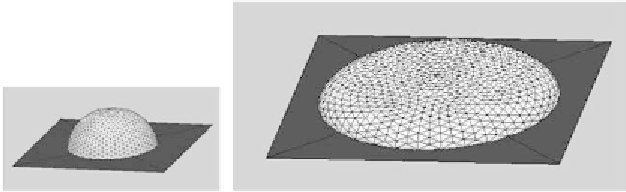
gravitational force flattens the drop on the solid surface. A numerical simulation of
the two shapes of droplets obtained with the numerical software Surface Evolver is
shown in Figure 3.46. The capillary length is of the order of 2 mm for most liquids,
Figure 3.46
Numerical simulations of a microdrop (
Bo
<<1) and a larger drop (
Bo
>>1) obtained
with Surface Evolver software (not to scale).