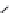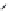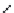Geoscience Reference
In-Depth Information
Figure 10.56 Electrolytic cell
assembly in the autoclave
[309]
.
Ag
Potentionstat
WE
RE2
RE1
CE
0.1
M
KCl
Ag/AgCl
(RE)
Liquid junction
0.5
M
A (OH)
2
(A = Ba, Sr)
Ti
Pt
Ti
(WE)
Pt
(CE)
0.5
M
A (OH)
2
(A = Ba, Sr)
regardless of the electrolysis conditions
[318]
. The preprocessed Ti substrate and
the platinum plate are suspended as the working electrode (anode) and the counter
electrode (cathode), respectively, by 0.5 mm diameter wires of the same metal as
the respective plates, keeping an interval of 30 mm between them in the electrolytic
cell containing 500 ml of the solution. The experiments were carried out at 150
C,
an approximate heating rate being 1.5
C/min. The Ti working electrode was polar-
ized potentiostatically from 50
C in the heating region to the end of the 150
C iso-
thermal region, keeping an anodic potential of
12.0 V versus Ag/AgCl. The
electrolysis current varies characteristically in the heating process and then remains
almost constant in the isothermal process. After the experiment, the Ti substrate
was washed in distilled water with an ultrasonic cleaner and dried at 120
C.
Kajiyoshi et al.
[29]
have also studied, in detail, the transport mechanism of film-
constituting elements. They propose two models: the first based on the principle
of tracer technique: (i) substitutional transport and (ii) interstitial transport. The sec-
ond is the short-circuit path model. The latter one is more appropriate here to describe
the mass transport in ATiO
3
(A
1
Ba, Sr).
Figure 10.57
illustrates schematically the
mass transport and film growth mechanisms of the short-circuit path model. In this
model, all the observed results can be explained comprehensively, together with an
atom-mixing mechanism accompanied by the dissolution
recrystallization process.
Figure 10.58
shows representative SEM micrographs of a fractured cross section of
5