Geoscience Reference
In-Depth Information
on solid surfaces has met with a fair degree of success
[249
251]
,asoppossedtothe
controlled assembly of nanoparticles in the organic solvent which has not been under-
stood precisely. In order to meet the major challenges in nanotechnology in realizing
many of the desired technological goals, self-assembly provides a possible convenient
route, but controlling size, size distribution, shape, and surface chemistries of the nano-
hybrid particles is critical to achieving desired structures. Initial syntheses have yielded
nearly spherical shapes due to the thermodynamic driving force of minimizing surface
area, and self-assembly has been limited to the close-packing of spheres
[252,253]
.
There are several reviews published on this aspect
[254,255]
. However, it is to be noted
that the self-assembly of hybrid organic
inorganic nanoparticles synthesized through
supercritical hydrothermal routes are seldom found in the literature.
The mechanism of making self-assembly structures involves two steps: The first
step is the positioning of perfectly dispersed particles (in solvent) on the substrate
by controlling interactions between substrate and particles' surfaces (hydrophilicity
and hydrophobicity). In the second step, capillary forces between the particles and
the surface laterally displace the particles during drying. Steric repulsion between
particles because of a capping agent on the surface prevents the aggregation of
nanoparticles. After complete evaporation in the third stage, an irreversible reorga-
nization of the particle
substrate interface occurs, which prevents the displacement
of the nanoparticles
[256]
.
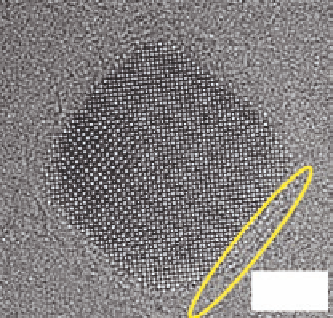
Figure 10.53
shows the HRTEM image of the self-
assembly ceria of nanoparticles obtained under supercritical hydrothermal condi-
tions in the presence of decanoic acid as the modifier. It is clearly seen from these
images that the organic ligand molecules are bonded to the surface of ceria nano-
cubes. With the increasing concentration of organic ligands, particle size not only
decreases but the morphology also changes from the cubic to the truncated octahe-
dron. The organic layer around the ceria particle is quite thin and it is shown with
a yellow loop.
Figure 10.54
shows the self-assembly of Co
3
O
4
and Fe
3
O
4
nanopar-
ticles obtained under supercritical hydrothermal conditions in the presence of
organic ligand molecules C
9
COOH and C
17
COOH, respectively. The decanoic acid
has a carbon chain length of 1.4 nm, and it can be seen that the distance between
Figure 10.53 HRTEM image of 7 nm size
decanoic acid modified ceria nanocubes
synthesized at 400
C and 30 MPa.
Source: Photograph courtesy of T. Adschiri.
Organic
molecules