Geoscience Reference
In-Depth Information
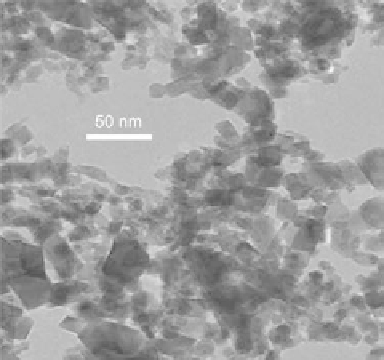
Figure 10.35 TEM image of LiMn
2
O
4
nanoparticles synthesized at 400
C and
30 MPa.
Source: Photograph courtesy of T.
Adschiri.
other methods. In the synthesis of LiMn
2
O
4
nanoparticles, oxidizing reaction atmo-
sphere has to be controlled by regulating oxygen gas partial pressure in the system.
In this case, Mn
2
1
of Mn(NO
3
)
2
should be oxidized into Mn
3
1
. In order to achieve
this, H
2
O
2
was fed into the system. H
2
O
2
decomposes at supercritical conditions
into oxygen gas, which forms a homogeneous phase with supercritical water to pro-
vide an excellent oxidizing atmosphere
[154
156]
. Ni nanoparticles have been
formed from nickel acetate and formic acid at 400
C and 30 MPa. In this case,
HCOOH was introduced as a reducing agent with nickel acetate solution
[157,158]
.
In supercritical water, HCOOH is decomposed into hydrogen and carbon dioxide.
An important point is that these gases and supercritical water form a homogeneous
phase, and this mixture of gas (H
2
and CO
2
) shows higher reducing ability than H
2
gas, as was reported in the literature
[159,160]
.
Although supercritical hydrothermal technology gives highly crystalline nano-
particles with a homogeneous composition, still there is a problem of larger size,
coagulation, and poor dispersibility of nanoparticles in aqueous solutions like water
as seen from
Figure 10.36
.
Poor dispersibility of such nanoparticles is due to the fact that there is an aggre-
gation between nanoparticles which leads to worse dispersion and, after a while,
precipitated particles will appear at the bottom of the bottles as shown in
Figure 10.36
. Hence, there is a trend to obtain small nanoparticles with a perfect
control over the size and morphology and high dispersibility with a modern
approach in materials processing like the use of organic ligands, capping agents,
surfactants, and chelating agents, which generate a new class of nanomaterials,
namely organic
inorganic hybrid nanomaterials. This strategy is based on the mis-
cibility of the organic ligand molecules with scH
2
O due to the lower dielectric con-
stant of the water; and the nanocrystal shape control by selective reaction of
organic ligand molecules to the specific inorganic crystal surface. For this purpose,
even some amino acids, peptides, proteins, or DNA are used to modify the particle
surfaces and such particles can be specifically combined with other proteins or