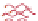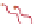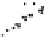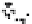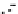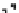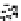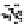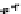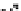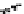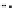Geoscience Reference
In-Depth Information
(001)
Nuclei
b
b
c
Figure 10.25 Shape control in ceria nanoparticle fabrication: (a) truncated octahedron in the
case when no organic ligand molecules are used; (b) at a low decanoic acid to ceria
precursor ratio, the preferential interaction of the ligand molecules with the ceria {001}
planes slows the growth of {001} faces relative to (111) faces, which leads to the formation
of nanocubes; and (c) at a high deconoic acid to ceria precursor ratio, organic ligand
molecules block growth on both (001) and (111) faces, which leads to the formation of
truncated octahedral and smaller crystals [44].
Figure 10.26 Mechanism of CeO
2
nanoparticles formation under
supercritical hydrothermal conditions.
Soluble intermediates
Crystals
Subcritical
(573
K)
CeO
2
c
rystals
Ce
3+
ion
Crystals
Subcritical
(673
K)
CeO
2
particles formation in scH
2
O. More or less a complete list of the materials
obtained under supercritical hydrothermal conditions is available in the works of
Reverchon and Adami
[58]
and Byrappa and Adschiri
[20]
. Basically, the hydro-
thermal synthesis method is available for the metal oxides by conventional hydro-
thermal synthesis method. The point to be noted here is that under supercritical
hydrothermal conditions, nanometer-sized metal oxides could be synthesized, and
crystallinity of the nanoparticles would be much higher when compared to the