Geoscience Reference
In-Depth Information
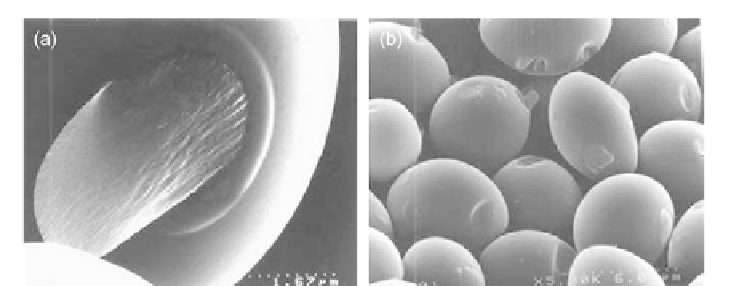
Figure 10.12 (a and b) SEM images showing fluid-like material coming out of solid
spherical and ovoid particles.
Source: Photographs courtesy of B. Basavalingu.
flows. We have also noticed different stages in the development of hollow and
porous carbon phase formation, i.e., fluid like material is coming out of the solid
spherical or ovoid particles through a vent and resulting in hollow ovoid or spheres
(
Figure 10.12a and b
).
Raman spectroscopic study of the run products indicates that the bulk of the car-
bon particles exhibit the broad spectrum with prominent peaks at 1350 and
1580 cm
2
1
, which are referred to D- and G-band of C
C stretching vibrations.
The G-band is from the carbon atoms forming hexagonal lattice, and the D-band is
associated with vibrations of carbon atoms in dangling bond in plane terminations
which is characteristic of sp
2
hybridization corresponding to graphitic or disordered
carbon material.
Figure 10.13
shows the typical representative Raman spectrum for
spherical and ovoid-shaped aggregates of carbon particles along with the Specpure
graphite sample. The Raman spectra obtained for a few selected scaly materials
having metallic luster and for the nanosized crystallites adhered to the inner walls
of the porous spherical particles is shown in
Figure 10.14
along with the spectrum
of commercially available diamond powder (Hyprez—Engis, USA). This spectrum
has a sharp peak at 1332 cm
2
1
and a shallow broad peak at 1590 cm
2
1
.
When chromium carbide was used as the source of carbon, under similar experi-
mental conditions, it yielded filamentous-type carbon along with some spherical
shaped carbon.
Figure 10.15
shows SEM images of filamentous carbon and spherical
particles.
Presser et al.
[68]
have studied in detail the thermodynamics of carbide reactions
with halogens in the formation of carbide-derived carbons—from porous networks
to nanotubes and graphene. Several carbides like SiC, Ti
3
AlC
2
, TiC, Ti
2
AlC, and
ZrC have been considered in such studies. Wang et al.
[83]
have studied the ther-
modynamics of diamond nucleation on the nanoscale. They conclude that the size
of the diamond-critical nuclei should be limited within several nanometers in the
hydrothermal synthesis and there should be a reduction of carbide (HSRC) super-
critical fluid systems
[84]
. According to the thermodynamic model,
they first