Geoscience Reference
In-Depth Information
Amongironoxides,Fe
2
O
3
has three modifications:
α
-Fe
2
O
3
(hematite type),
β
-Fe
2
O
3
,and
γ
-Fe
2
O
3
(maghemite type) and some authors even predict the mono-
clinic phase
-Fe
2
O
3
. There are various methods of synthesizing iron oxides, and
the simplest way is forced hydrolysis of Fe
3
1
salt solutions. But the good-quality
crystals are obtained by the hydrothermal method or to some extent the flux
method. Several authors
[97
ε
100]
have carried out the preparation of fine crys-
tals of magnetite.
-Fe
2
O
3
(hematite) is
stable up to about 550
C. Above this temperature, up to 710
C, mixtures of
α
In the pure Fe
2
O
3
a
H
2
Osystem,
α
-Fe
2
O
3
and Fe
3
O
4
are stable. Runs above 710
C yield magnetite (Fe
3
O
4
)asthe
only stable phase. Viswanathiah et al.
[97]
have conducted experiments in the
temperature interval 100
600
C. Traces of magnetite are found to be stable even
temperatures as low as 100
C. From 100
Cupto200
C,
at
iron formate
(C
2
H
2
FeO
4
-Fe
2
O
3
) are the major phases along with
traces of magnetite. HCOOH is used as the mineralizer, and it contributes to the
formation of siderite phase also. Further, the liberation of carbon monoxide into
the system creates a reducing environment, which promotes the stabilization of
Fe
3
O
4
rather than of Fe
2
O
3
. Thus, perfect octahedral crystals of magnetite are
formed at 250
C.
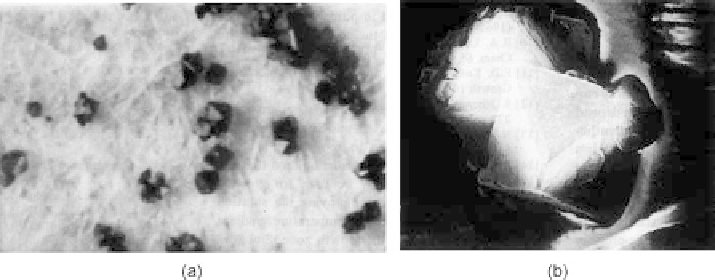
Figure 9.20
shows hydrothermally grown magnetite crystals, as
well as aggregates.
Although there are several methods of preparing crystalline iron oxide powders,
hydrothermal and sol
2H
2
O) and hematite (
α
gel techniques are supposed to be the most popular ones.
Hirano and Somiya
[99]
have obtained magnetite crystals under hydrothermal con-
ditions in the presence of hydrogen. The hydrogen for the control of the reduced
atmosphere was supplied by the reaction of charged metallic iron with water
[100]
.
The experiments were carried out at 550
C and 1000 kg/cm
2
. As the concentration
of hydrogen was increased, the (110) faces of magnetite were found to predominate
over the (111) faces. The authors have used 10 M NaOH as the solvent. No hema-
tite formation was reported. Dendritic magnetite crystals were grown in 5 M
Figure 9.20 Hydrothermally grown magnetite crystals: (a) Magnetic crystals along with
other products of the system; (b) magnified image of magnetite crystals.