Geoscience Reference
In-Depth Information
The experiments done in platinum-lined autoclaves showed that platinum
dissolves in hydrohalogenide complexes; hence, the process of dissolution of TeO
2
in the acid deteriorates and goes out of control. Thus, effective usage of hydrother-
mal technology of paratellurite could not be developed owing to the difficulties in
observing and controlling the parameters which determine the crystallization of
α
-TeO
2
in platinum-lined steel autoclaves.
Popolitov et al.
[95]
have studied the solubility of paratellurite in detail in
hydrochloric acid, which is an effective solvent for the recrystallization of TeO
2
[94]
.
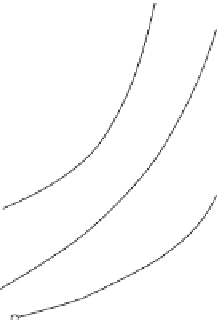
Figure 9.18
shows the dependence of solubility of tellurium dioxide and HCl
concentrations measured by titration with sodium hydroxide. The starting material
used was monocrystals of paratellurite obtained through spontaneous crystallization
from water solutions of HCl.
The hydrothermal growth of monocrystals on seeds is known to include the
following stages: Dissolution of solid substance, transport of dissolved materials to
the growth region, and the growth of seed crystals. To reveal the mechanism of
transport, it is of great importance to use the data on solubility of paratelluride in
HCl solutions and to consider the possible mechanism of transport from the disso-
lution region to the growth region in the process of
-TeO
2
growth on the seed.
The synthesis of paratellurite with 100% output occurs in aqueous solutions of
nitric acid with concentrations up to 25% at temperatures of 260
α
340
C and pres-
sures of 50
370 atm
[91]
. Element tellurium serves as the nutrient, so that the
synthesis reaction occurs as follows:
3Te
4HNO
3
!
3TeO
2
ð
solid
Þ
1
2H
2
O
4NO
m
ð
9
:
7
Þ
1
1
Figure 9.18 Dependence of solubility of tellurium
dioxide and HCl concentration
[94]
.
2
1
0
1
2
3
C
HCL
(wt%)