Geoscience Reference
In-Depth Information
taken with K
2
CO
3
solution. However, when the concentration of these components
in the nutrient is higher than 1.6% in 10% Na
2
CO
3
solution, crystals practically do
not grow. Kuznetsov and Shternberg
[72]
have investigated the crystallization of
ruby under hydrothermal conditions by three methods: (1) Recrystallization of alu-
minum oxide in solutions containing chromium; (2) Joint recrystallization of Al
2
O
3
and Cr
2
O
3
, which are placed in the autoclave as separate components; and (3) finally,
recrystallization of ruby crystals having a given chromic oxide concentration. The
last method makes possible the uniform and controlled introduction of the impurity
into the solution and the grow
in
g crystal throughout the experiment.
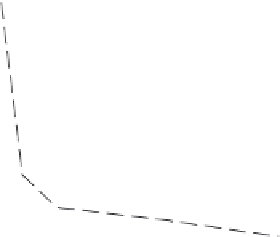
Figure 9.13
shows the growth rate of the
face versus the Cr
2
O
3
concentration in the initial
charge; 10% Na
2
CO
3
solution at 550
C, autoclave 60% filled. These authors con-
clude that in carbonate and bicarbonate solutions, chromic oxide and aluminum
oxide have substantially different solubilities in carbonate solutions and different
rates of dissolution. Also, the effect of solution redox potential on the transport of
chromic oxide under hydrothermal conditions has been studied by these authors.
Laudise and Ballman
[73]
and Monchamp et al.
[74]
have carried out very large-
scale growth of sapphire and ruby with considerable success.
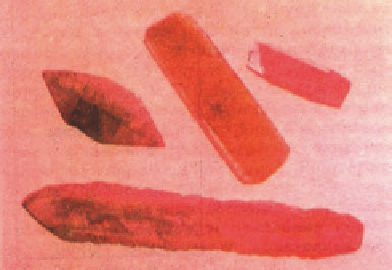
Figure 9.14
shows
hydrothermally grown ruby crystal
[75]
. Green crystals containing iron have been
ð
1011
Þ
Figure 9.13 Growth rate of
ð
1011
Þ
face
versus Cr
2
O
3
concentration in the initial
charge
[72]
.
C' (wt%)
1.6
1.2
0.8
0.4
0
0.2
0.4
0.6
0.8
1.0
V (mm/day)
Figure 9.14 Hydrothermally grown
ruby crystals.
Source: Courtesy of Balitskii
[75]
.