Geoscience Reference
In-Depth Information
In the growth of zincite, the use of alkalis sometimes leads to the formation of
hydrogen arising as a result of the oxidation of the autoclave material. The amount
of hydrogen formed depends greatly on the temperature, the concentration of the
alkali, and the duration of the experiment. In fact, the dissolution of the crystals,
the transport processes, and crystallization in turn depend greatly on the redox
potential of the medium. Sakagami
[59]
has obtained zincite crystals of high purity
by the hydrothermal method under a partial pressure of oxygen, using platinum-
lined autoclaves and ultrapure reagents. The growth conditions are as follows:
400
C
Growth temperature
370
10
15
C
Temperature difference
Total pressure
700
1000 kg/cm
Partial pressure
10
30 kg/cm
Solvent
KOH 3.0 M
1
LiOH 1.5 M
Oxidizer
H
2
O
2
0.1
0.3 M
Nutrient
ZnO sinter
Lining tube
Point (0.3 mm thickness)
Growth run
15
20 days/run
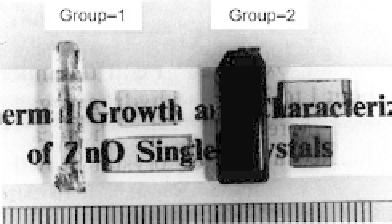
Figure 9.7
shows high-purity crystals of zincite o
bt
ained at a growth rate of
0.2 mm/day in a direction normal to a prismatic face
The degree of colora-
tion depends upon the growth conditions, e.g., solutions containing a H
2
O
2
oxidizer
and without an oxidizer. The author has used the coulometric technique to detect a
small deviation in the stoichiometric constituent toward Zn
1
1
x
O. The concentration
of excess Zn in the crystals grown under excess partial pressures of oxygen
decreased to the minimum of 0.8 ppm, and their resistivity increased to about
10
8
ð
1010
Þ:
m. Thus, obtaining the composition of the solution in which the zincite crys-
tallizes and changes the habit and properties of the developing crystals. In KOH
solutions, ZnO crystals or prismatic habits, drawn out sharply along the c-axis, are
formed in caustic soda solutions. The crystals are more isometric in LiOH solu-
tions. The growth rate of the monohedral face is very slow, while that of the pyra-
mid face is commensurable with or slightly exceeds that of the prism, and the
crystals assume a tabular form. The presence of F
1
ions in the solution increases
the growth rate of the monohedral (0001) face. If the solution contains iron, charac-
teristic zincite twins grow along the face of
Ω
the monohedron.
In aqueous
Figure 9.7 High-purity crystals of
zincite obtained at a growth rate of
0.2 mm/day
[59]
.