Geoscience Reference
In-Depth Information
HgSe
2HCl
!
H
2
Se
HgC
12
ð
8
:
43
Þ
1
1
HgTe
2HCl
!
H
2
Te
HgCl
2
ð
8
:
44
Þ
1
1
Table 8.4
gives the optimum conditions for the crystallization of HgSe and
HgTe.
In the hydrothermal synthesis of FeTe
2
, FeSe
2
, NiSe
2
, NiTe
2
, CoSe
2
, and CoTe
2
crystals, Na
2
Se and Na
2
Te are used as the nutrient materials. The synthesis of dise-
lenide and ditelluride of Ni and Co is carried out in quartz ampoules, whereas the
synthesis of diselenide and ditelluride of iron is carried out in titanium ampoules or
liners.
The physical properties of these hydrothermal selenides and tellurides are much
superior as they are grown in the controlled atmosphere.
The solvothermal and hydrothermal growth of tellurides and selenides as nano-
size crystals carries a special interest in recent years owing to their application
potential in various technological fields.
The hydrothermal growth of niobates and tantalates began during the 1960s, at
the time when the hydrothermal synthesis of inorganic compounds without natural
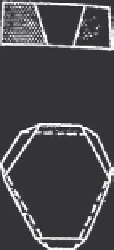
(a)
(111)
(112)
(111)
(111)
(121)
(011)
(211)
(b)
(111)
(111)
(011)
(112)
(111)
(111)
(110)
(111)
(111)
(111)
(211)
(111)
(111)
(110)
(c)
(d)
Figure 8.31 Morphology of ZnSe in NaOH and LIOH solutions
[111]
.