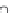Geoscience Reference
In-Depth Information
the monophase synthesis of the above compounds, the surplus amount of the nutri-
ent is taken. The Cu
2
S is abundant in nature as chalcogenides. The other univalent
metal sulfides are not common in the literature.
8.11.2 Hydrothermal Synthesis of Divalent Metal Sulfides
Divalent metal sulfides form the largest group of sulfides obtained by the hydro-
thermal technique. A large number of sulfides like ZnS, CdS, HgS, PbS, MnS, FeS,
Fe
3
S
4
, NiS, CoS, and CuS have been obtained within a wide range of pH
[93,96,98]
. Kuznetsov
[98]
has studied the solubility and stability fields of these
sulfides under hydrothermal conditions in detail.
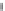
Figure 8.26
shows the solubility
of HgS, ZnS, CdS in KOH and NaOH solutions
[98
100]
.
In all cases the dependencies are nonlinear. This means that the composition of
complexes is changed with an increase in the solvent concentration. The analysis
m
HgS
lg
m
HgS
-1.5
0.08
—0.54
m
NaHS
—1.04
m
NaHS
—1.43
m
NaHS
—2.11
m
NaHS
×
0.06
-2.0
0.04
×
×
0.02
-2.5
×
×
0
lg
m
HS
-
-0.4
0
+0.4
9
10
11
p
H
lg
m
ZnS
-1.0
8
6
-1.5
4
-2.0
2
0
20
40
-2.5
-0.5
C
NaOH
(%)
lg
m
OH
-
0
0.5
Figures 8.26 Solubility of HgS, ZnS, CdS in KOH and NaOH solutions
[98]
.