Geoscience Reference
In-Depth Information
are highly useful as selective gas absorbers, catalysts, and so on. Hence, the hydro-
thermal and solvothermal syntheses of nanostructures of these compounds carry a
greater significance in recent years than the growth of bulk single crystals. Here we
discuss only a few representative compounds and their physical properties.
Among the fluorocarbonates, KCu(CO
3
)F and BaCu(CO
3
)F
2
exhibit excellent
magnetic properties.
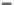
The variation of the reciprocal susceptibility of KCu(CO
3
)F, in the temperature
range 4.2
300 K, is given in
Figure 8.9
. The magnetic susceptibility is weak and
nearly independent of the temperature between 300 and 100 K, it then increases
continuously from 100 to 4.2 K.
Strong antiferromagnetic interactions must be present between nearest Cu
2
1
neighbors in order to explain the magnetic behavior at high temperature; however,
this hypothesis does not appear really acceptable for two reasons:
1. Superexchange interactions Cu
a
F
a
Cu possible in the infinite files along a are not gener-
ally very strong (J/k
100 K).
2. Super-superexchange interactions Cu
a
O
a
O
a
Cu (through the carbonate ions) are proba-
bly even weaker.
35
1/X
g
×10
-4
30
25
20
15
10
5
Temperature (K)
0
0
50
100
150
200
250
300
Figure 8.9 Reciprocal susceptibility of KCu(CO
3
)F
[32]
.
Source: Courtesy of the Gauthier-Villars/ESME, Paris.