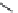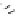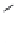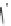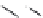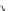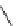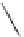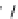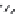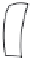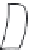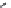Geoscience Reference
In-Depth Information
(a)
(b)
70 % fill
3
M HCOOH
65 % fill
2.5
M HCOOH
(c)
(d)
(e)
60 % fill
2
M HCOOH
55 % fill
1.8
M HCOOH
50 % fill
1.5
M HCOOH
Figure 7.86 Schematic diagram of morphological variations
[363]
.
and the availability of isotopically pure starting materials help in getting highly
pure crystals. Further, the temperature and pressure condition involved in the
growth of Li
2
B
4
O
7
is relatively low and this insists upon very simple autoclave
designs and higher practicability. Byrappa et al.
[363]
have studied the solubility of
Lithium Triborate, LiB
3
O
5
(LBO) under hydrothermal conditions with reference to
the molarity, % fill, pressure, etc. in different solvents. The solubility is found to
be positive. The solubility measurements were carried out by the weight loss
method in different solvent media and these studies were performed with utmost
care to obtain good results by taking the average values. The possible errors, if
any, in our measurements are due to the fluctuations in temperature (
1
C), weight
6
and volume determination.
Figure 7.87a
c
shows the solubility curves for LBO
crystals with varying molarity of LiOH, HCOOH, pressure, and temperature. As
evident from
Figure 7.87
, the ideal temperature for the growth of LBO crystals is
within the temperature range of 200
260
C(
Figure 7.87a
). Similarly, the ideal
molarity range of the mineralizer in the growth of LBO crystals is 1.5
1.8 M
(
Figure 7.87b
).
Li
3
B
5
O
8
(OH)
2
. The synthesis of Li
3
B
5
O
8
(OH)
2
crystals has been reported earlier
by the authors while investigating the system Li
2
O
a
ZnO
a
B
2
O
3
a
H
2
O under