Geoscience Reference
In-Depth Information
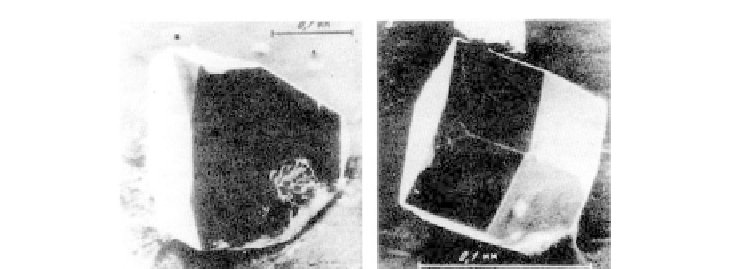
Figure 7.83 Characteristic photographs of Li
2
B
4
O
7
crystals
[350]
.
same time a Japanese group has reported the preparation of Li
2
B
4
O
7
thin films by
the sol
gel method
[343]
. The Li
2
B
4
O
7
crystals obtained by the Czochralski
method always show coring which is the main macrodefect thereby hindering wide
industrial applications of Li
2
B
4
O
7
in the manufacture of SAW and BAW devices, i.
e., a continuous or intermittent opaque region parallel to the growth direction at the
central core of the crystal often occurs and is related to the growth rate, deviation
of the melt composition from stoichiometry (e.g., becomes Li rich) and contents of
H
2
OorO
2
in the melt of Li
2
B
4
O
7
[358,359]
. In the Bridgman growth of Li
2
B
4
O
7
,
the authors have observed a similar opaque core in the Li
2
B
4
O
7
crystal and consid-
ered this defect to be a cluster of holes and a cellular structure or dendritic
[360]
.
A few white inclusions of impurities and evidence of constitutional supercooling in
the holes or on the walls of cells have been observed. Similarly, the cracking by
thermal stress frequently occurs in Cz growth because of the anisotropy of the
expansion coefficient of Li
2
B
4
O
7
. No cracks due to thermal stress occur in the
Bridgman growth of Li
2
B
4
O
7
for various orientations, since the temperature gradi-
ent in the Li
2
B
4
O
7
crystal is smaller. However, if the residual melt on the top of an
as-grown crystal in
directions changes into polycrystalline phase by
fast cooling, cracks in the Li
2
B
4
O
7
occur often in the top region. Similarly, the
sol
h
100
i
or
h
110
i
gel technique has some disadvantages although the method has several advan-
tages like: It allows high purity, low processing temperature, precise composition
control, and the formation of bulk monoliths, fibers and coating films. The impor-
tant conclusions drawn by the authors based on their work on sol
gel method is
that a larger amount of water is necessary for the crystallization of Li
2
B
4
O
7
thin
films, but also to suppress the formation of undesirable crystalline phases other
than Li
2
B
4
O
7
[361]
. Obviously, hydrothermal technique helps not only in the crys-
tallization of Li
2
B
4
O
7
crystals at fairly lower temperatures, but also in solving
many of the problems encountered in other methods as described earlier. Contrary
to the result of Garret et al.
[346]
, no difficulties were experienced using platinum
crucibles to contain the melt by the authors
[359]
. Hence, in order to avoid these
controversies, Byrappa and Shekar
[362]
used Telfon liners such that the incorpo-
ration of impurities could be totally avoided.