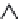Geoscience Reference
In-Depth Information
Figure 7.75 Solubility curve of Nd:YVO
4
[321]
.
25
20
15
10
5
0
50
150 250
Temperature (°C)
350
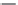
Figure 7.76 Arrhenius plot log S
versus 10
3
/T.
1.4
1.2
1
0.8
0.6
0.4
3
4
5
10
3
/
T
6
7
the solubility does not alter much, which suggests that the crystal growth beyond
this temperature is not practical. Hence, from the solubility data, it is found that the
temperature range between 180
and 300
C is the most ideal, and 240
C particu-
larly is the best growth temperature. The solvents like HCl, HNO
3
,H
2
SO
4
, and
HCOOH when used alone were not good solvents since fine crystalline products
having no morphology were obtained. The use of alkaline solvent did not result in
the crystallization of yttrium orthovanadates but instead yttrium hydroxides, Y
(OH)
3
, or hydrous yttrium vanadates, YVO
4
nH
2
O, were formed. Since the use of
single acid solvents did not substantially enhance the solubility, mixed acid minera-
lizers were tried (HNO
3
1
HCl, HCl
1
H
2
SO
4
, HNO
3
1
HCOOH, and so on). It
was found that 1.5 M HCl
3.0 M HNO
3
taken in 2:1 ratio is the best solvent for
the growth of R:YVO
4
crystals.
Rare earth orthovanadate, Nd:YVO
4
, crystals were obtained under hydrothermal
conditions. The earlier workers used mostly the melt technique to obtain rare earth
vanadates. The study of the morphology of crystals is very important since the crys-
tal habit is governed by the kinetics rather than the equilibrium considerations with a
number of factors like degree of supersaturation, solvent type, and the pH of the
media
[336]
. Thus, the authors have studied the influence of these parameters on the
growth morphology of Nd:YVO
4
and Eu:YVO
4
. The most commonly observed
1