Geoscience Reference
In-Depth Information
hydrothermal origin
[299,300]
. Similarly, ionic conductivity has been reported in
some of the crystals of MRP
4
O
12
[301]
. However, the ionic conductivity values are
not high and not comparable to mixed valent metal phosphates. Since the majority
of these compounds either belong to the NASICON family or are analogous to
NASICON structures,
they
obviously
exhibit
high
ionic
conductivity.
Na
3
M
2
1
(PO
4
)
3
(M
Sr, Mg, Fe, Mn); A
3
M
3
1
(PO
4
)
3
(A
Li, Na, Ag, K; M
Cr,
5
5
5
Fe); ABC(PO
4
)
3
(A
Mn,
Co, Ni, Zn, Pb, Cd); (Na.
66
Zr.
33
)
2
(P
2
O
7
)
2
, and so on, exhibit high ionic conductiv-
ity. The unique feature of these NASICON analogs is the thermal stability. Unlike
the NASICON compound, whose ionic conductivity is hampered by the presence of
multiple phase transitions from greater than 60
C, these compounds show only one or
two phase transitions, usually greater than 400
C
[302]
.
Figure 7.70
shows the
Differential Thermal Analysis (DTA) curves for the representative superionic pyro-
phosphates. Similarly MSn
2
(PO
4
)
3
(M
K, Rb, Cs; B
Ca, Sr; C
Ln and Bi) NaHMP
2
O
7
(M
5
5
5
5
Na, K, NH
4
) grown hydrothermally is
stable up to 1200
C without phase transitions
[303
5
305]
. Also, some of the rare earth
phosphates show an ultra-low thermal expansion coefficient, which has a potential
application in several fields
[306]
. Byrappa et al.
[307]
) and Sanjeev
[308]
have stud-
ied the ionic conductivity in superionic pyrophosphates within a wide range of
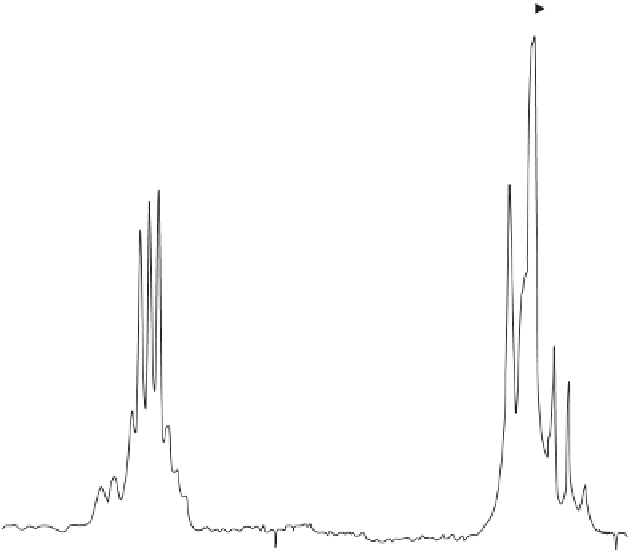
4
F
3/2
4
I
9/2
4
F
3/2
4
I
11/2
Cs Nd (PO
3
)
4
T
= 77
K
1100
1000
900
λ
(nm)
Figure 7.69 Luminescence spectra of
α
-CsNd (PO
3
)
4
crystals
[300]
.