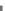Geoscience Reference
In-Depth Information
100
100
80
80
60
60
40
40
20
20
10
20
30
40 50
P
2
O
5
(wt%)
60
70
80
90
0
2.5
5
7.5
10
12.5
15
17.5
H
2
O (wt%)
(a)
(c)
100
80
60
40
20
6
8
10 12
Na
2
O (wt %)
14
16
18
(b)
Figure 7.66 Growth rate of superionic pyrophosphate, NaHCo(P
2
O
7
) versus (a) P
2
O
5
concentration, (b) Na
2
O concentration, and (c) H
2
O concentration
[298]
.
varies with the above-mentioned growth parameters. These experiments indicate that
the degree of supersaturation, concentration of cations, and dopants determine the
morphology of these pyrophosphates. Such studies help in the successful growth of
superionic phosphates in the form of single crystals with a desired morphology. They
help us to understand the growth technology of these phosphates in detail.
A number of factors such as the degree of supersaturation, temperature, pressure,
accidental/deliberate entry of impurities and their concentration, type of the solvent,
and pH of the mineralizer affect the habit of crystals obtained under hydrothermal
conditions. Habit modifications are also observed when significant changes in the
growth temperature occur, since an increase in temperature hastens the growth kinetics
andinturngrowthrate.
The crystal faces of most of the superionic pyrophosphates are more or less smooth
and vitreous in luster with a high degree of transparency. The morphology of these
pyrophosphates varies from one another depending upon the cations present.
It is interesting to observe the morphological variations in the pyrophosphates
with reference to the variation in the cation. The cations used are essentially the