Geoscience Reference
In-Depth Information
7.14 Hydrothermal Growth of Mixed Valent
Metal Phosphates
The interest in the synthesis of phosphates, other than rare earth phosphates, is con-
nected with the publication of NASICON, whose ionic conductivity is equivalent
to that of Na
-alumina. It has a mixed framework structure and the general for-
mula is Na
1
1
x
Zr
2
Si
x
P3
2
x
O
12
;O
β
3
[265]
. It is a solid solution between
NaZr
2
P
3
O
12
and Na
4
Zr
2
Si
3
O
12
. NASICON posed a challenge to materials scientists
in understanding its structure and conduction mechanism due to the lack of single
crystals, nonstoichiometry in the composition, zirconium deficiency, and so on.
Many variations have been investigated by appropriate substitutions in the
NASICON system
[261]
. The basic structure of most of these derivatives remains
that of Na
3
Sc
2
P
3
O
12
. NASICON has two structure types, namely, NASICON and
anti-NASICON
[266,267]
.
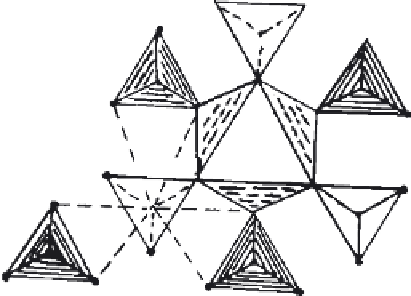
The structure of NASICON is highly complicated because of its solid solution
nature. There are nearly a hundred atoms per unit cell, which makes it rather diffi-
cult to describe. However, simplification can be introduced by assuming that this
type of structure is derived from that of glaserite K
3
Na(SO
4
)
2
—a rhomobohedral
super-structure
[268]
(
Figure 7.62
).
Hong
[266]
studied the structure of NASICON, Na
1
1
x
Zr
2
Si
x
P
3
2
x
O
12
, solid solu-
tion series and reported a monoclinic deformation. Space group is C
2
/c in the
region of x
5
x
,
,
2.2. The first crystal structure of NASICON-type material was
performed in 1968 by Hagman and Kierkegaard
[269]
, who studied the structure of
NaM
2
(PO
4
)
3
(where M
1.8
Ge, Ti, and Zr) and found them to be isomorphous.
Na
4
Zr
2
(SiO
4
)
3
structure has been further studied by Sizova et al.
[270]
and Tranqui
et al.
[271]
and reported multiple Na
1
sites leading to the nonstoichiometry in
NASICON compound. Delbecq et al.
[272]
, Susman et al.
[273]
, Collin et al.
[274]
, Boilot et al.
[275]
, Clearfield et al.
[276]
, and Baur et al.
[277]
have reported
five different lattice sites for Na
1
, out of which only the Na
1
is mobile.
Stoichiometry, structure, and conductivity of NASICON are being studied by
several authors consistently from time to time. Since the structure of NASICON is
5
Figure 7.62 Structure of glaserite
K
3
Na (SO
4
)
2
[268]
.
K(0)
K(1/2)
Na(1/2)