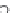Geoscience Reference
In-Depth Information
ZrO
2
1 — Na
2
ZrGe
2
O
7
2 — Na
4
Zr
2
Ge
5
O
16
3 — Na
4
Zr
2
Ge
3
O
12
4 — Na
2
ZrGeO
5
5 — Na
3
HZrGe
2
O
8
6 — Na
3
HGe
7
O
16
·4H
2
O
7 — Na
4
Ge
9
O
20
8 — Na
2
GeO
3
·H
2
O
ZrGeO
4
3
4
2
1
5
6
7
8
GeO
2
mol%
Na
2
O
Figure 7.50 Crystallization field of sodium zirconium germanates in the system
Na
2
O
a
ZrO
2
a
GeO
2
a
H
2
O
[95]
.
(a)
(b)
1:1
1:1
ZrO
2
+ Na
2
ZrGe
2
O
7
ZrO
2
Na
3
HZrGe
2
O
8
(III)
1:2
1:2
(II)
1:4
1:4
Na
4
Zr
2
Ge
5
O
16
I
I
+
I + III
(I)
II
1:6
1:6
III + II
0
10
20
C
NaOH
, wt%
30
40
0
10
20
C
NaOH
, wt%
30
40
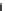
Figure 7.51 Crystallization field of sodium zirconium germanates in the system
Na
2
O
a
ZrO
2
a
GeO
2
a
H
2
O at 300
C (a); and at 500
C (b)
[95]
.
germanium oxides. The sequence of germanate formation in the hydrothermal
Na
2
O
a
ZrO
2
a
GeO
2
a
H
2
O system may be
represented by the
scheme
in
Figure 7.52
.
The formation of NaZr-germanates is accompanied by a decrease in the coordi-
nation numbers of Zr from 8 to 6 in all NaZr-germanates. If we pass from alkali-
free to alkali-zirconium germanates, the GeO
2
/ZrO
2
ratio first increases in a jump-
wise manner and then decreases to the initial minimum value equal to unity:
ð
q
Þ
1
!
2
:
5
!
2
!
1
:
5
!
1
ð
7
:
14
Þ
A decrease in the GeO
2
/ZrO
2
ratio is reflected in a decrease of the condensation
of germanium
oxygen tetrahedra: