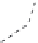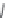Geoscience Reference
In-Depth Information
Figure 7.39 NC diagram of crystallization
in the system Na
2
O
a
BaO
a
SiO
2
a
H
2
O
[194]
.
N(BaO/SiO
2
)
6:1
BaSiO
3
2:1
Ba
4
[Si
6
O
16
]
20
40
C
NaOH
(mac%)
001
010
110
203
111
456
012
122
122
010
051
021
031
055
001
105
105
011
012
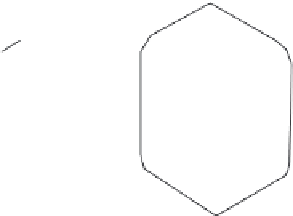
Figure 7.40 Schematic diagram of the crystal morphology of the barium silicates
[182]
.
quartz depends only on the concentration of Ba(OH)
2
(M
Ba
O). All the transitions to
NaBa
3
Si
2
O
7
(OH) are controlled mainly by the concentration of NaOH. Mn
2
1
-
doped BaSi
2
O
5
shows luminescence.
The other important system Na
2
O
H
2
O gives the following phases
under hydrothermal conditions: Mn
7
SiO
12
(braunite), Mn(OH)
2
(pyrochroite),
Mn
2
SiO
4
(tephroite), Na
2
Mn
7
Si
8
O
24
,Na
2
Mn
6
Si
7
O
21
,Na
2
Mn
3
Si
4
O
12
,Na
2
Mn
2
Si
2
O
7
,
Na
2
MnSiO
4
,Na
6
Mn
3
Si
6
O
18
,Na
2
MnSi
8
O
18
,Na
2
Mn
7
(SiO
3
)
8
,Na
2
Mn
8
O
16
a
MnO
a
SiO
2
a
nH
2
O.
Several of them occur in nature as minerals.
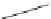
Figure 7.41
shows the NC diagram of
crystallization of the system Na
2
O
H
2
O
[195]
. Most of these phases
belong to the lower symmetry classes (monoclinic and orthorhombic) and some of
them are excellent luminescent materials. Further, these compounds crystallize at
T
a
MnO
a
SiO
2
a
400
C and MnO/SiO
2
.
1. These compounds can be obtained using alkaline
.
solutions, NaOH (
20%). The major difficulties concerning this system are associ-
ated with the change in the valency of Mn
2
2
to Mn
4
1
[196]
.
A majority of these sodium manganese silicates—Mn
2
[SiO
4
], Na
2
Mn
2
[Si
2
O
7
],
Na
2
Mn(SiO
4
), Na
2
Mn
7
(SiO
3
)
8
,Na
6
Mn
3
(Si
6
O
18
)—show useful physical properties.
,