Geoscience Reference
In-Depth Information
9
B — Brucite
E — Enstatite
F — Forsterite
P — Periclase
Q — Quartz
S — Serpentine
T — Talc
V — Water vapor
8
50
45
7
40
6
35
II
III
IV
I
5
30
4
25
Q
Q
Q
Q
Q
T
T
E
E
T
E
E
T
E
20
S
3
S
F
F
V
P
V
BP
V
P
VB
P
V
BP
15
2
10
1
5
300
400
500
600
700
800
900
Temperature (°C)
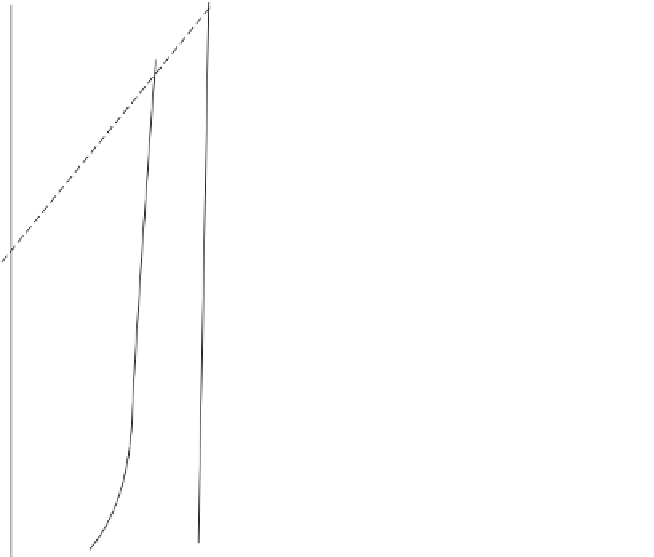
Figure 7.25 Pressure
temperature curves of univariant equilibrium in the system
MgO
a
SiO
2
a
H
2
O
[112]
.
Felspars: The high-temperature varieties of felspars have been successfully pre-
pared by the end of the nineteenth century. However, the complexities in their
forms and intergrowth were realized only during the 1950s.
Several hydrous and anhydrous aluminosilicates have been synthesized for the
past 150 years using hydrothermal technique. Among these, the dense anhydrous
aluminosilicates carried a great significance because of their role in natural meta-
morphic processes. Though several minerals—like garnets, sillimanite, analusite,
kyamte, and epidotes—have been synthesized using hydrothermal method, no
reproducible synthesis of these minerals was achieved before the work of Coes
[128]
, whose works are briefly described here for the benefit of the readers in
Table 7.14 [120]
.
The other important minerals synthesized during the nineteenth century by
hydrothermal method are staurolite, chloritoid, beryl, vesuvianite, lanosonite, ber-
trandite, lowsonite, bertrandite, low-temperature forms of the lithium aluminosili-
cates, spodumene, eucryptite, petalite, micas, clays, tourmaline, uranium minerals,
and so on. Therefore, we describe the synthesis of only some technologically
important silicates under hydrothermal conditions.