Geoscience Reference
In-Depth Information
Na
5
Sc Si
4
O
12
,Na
5
Er Si
4
O
12
, etc. has been reported in the literature. The crystal
structure of Na
5
RSi
4
O
12
was reported by Maksimov et al.
[43]
and it is shown in
Figure 7.4
. Thus, the importance of the rare earth silicates was established during
the 1980s. Here, we discuss the behavior of various rare earth silicates and alkali
rare earth silicate systems under hydrothermal conditions within a wide range of
pressure
temperature conditions. Also, the role of different mineralizers and the
starting molar ratios on their crystallization has been studied to obtain a general
picture, instead of describing each and every rare earth silicate compound and its
synthesis.
In the carbonate solutions and in mixed solutions of NaOH
Na
2
CO
3
, two new
1
phases crystallize and when RE
2
O
3
:SiO
2
5
1:4, NaRSi
6
O
14
crystallizes, where
R
Eu. These crystals are quite stable in pure Na
2
CO
3
solution and NaOH up
to 10%. With an increase in the concentration of the solutions, apatite like phase
forms, and with a decrease in the concentration of the solutions, the stability of
NaRSi
6
O
14
falls along with the stability field of quartz. In a pure carbonate solu-
tion, together with NaRSi
6
O
14
, quartz always crystallizes. With an addition of
NaOH, the quantity of quartz reduces but does not disappear totally.
When R
2
O
3
:SiO
2
La
5
approaches
1:1,
the
system gives
another
phase
Na
3
RSi
6
O
15
2H
2
O, which is the characteristic phase for the entire rare earth series,
and it persists up to the concentration of NaOH
30%, and beyond 30% of NaOH,
it separates into two types: Na
3
R[Si
2
O
7
] for the second half of the series (yttrium
series) and Na
2
R[SiO
4
](OH) for the first half of the rare earth series (lanthanum
series). An overall scheme of crystallization shows that when mixed mineralizers
(NaOH
5
N
2
CO
3
) are used, the crystallization pattern changes and is shown for the
Nd representative in
Figure 7.5 [44]
It is interesting to see that with a decrease in
the amount of H
2
O in the alkali-carbonate system, the field of Na
3
RSi
6
O
15
1
2H
2
O
also decreases and only one phase NaR
6
O
14
crystallizes. In the NaOH
Na
2
CO
3
1
600
C and P
solution and at T
2 kbar and only in experiments with
NaF and NaCl, it is shown that the surplus silica for the lighter rare earth series in
the system, only NaRSi
6
O
14
crystallizes in all cases, of course,
550
1
5
2
5
2
the crystal
y
y
a
1/2
b
1/2
2/3
x
x
5/6
1/2
1/2
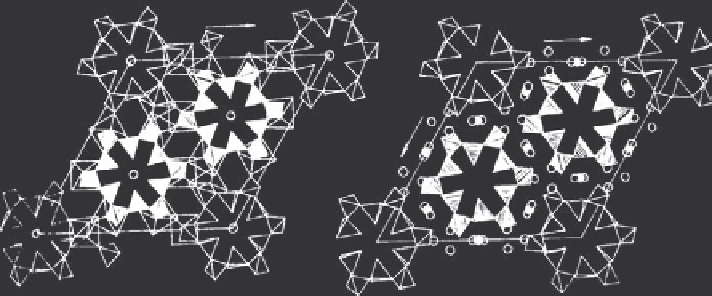
Figure 7.4 Crystal structure of Na
5
RSi
4
O
12
[43]
.