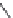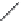Geoscience Reference
In-Depth Information
(a)
001
001
201
012
111
101
012
011
011
201
0
10
100
011
100
011
012
201
201
012
112
101
111
210
110
210
(ii)
400
μ
(i)
500
μ
211
001
0
11
201
100
010
011
210
211
201
(iii)
200
μ
(b)
(c)
001
111
001
111
101
011
011
101
010
110
010
1
0
0
100
010
110
110
100
(i)
(ii)
(i)
(ii)
Figure 6.22 (a) Crystal of
β
-eucryptite at (i) 500
C, (ii) 750
C, and (iii) 850
C. (b) (i) SEM
and (ii) schematic diagram of natrolite. (c) Crystal morphology of (i) thomsonite and (ii)
edingtonite
[119]
.
sodalite
[40]
. In fact, the exploration in hydrothermal systems of reactions yielding
salt-bearing sodalites began nearly 130 years ago
[126,127]
.CarlgrenandCleve
[127]
made sodalites from kaolinite and aqueous caustic soda containing the relevant dis-
solved salts at temperatures ranging about from 185
Cto220
C. Sodalite can also be
made very easily in the absence of any salts; if made so then they contain only zeolitic
water and a limited amount of soda. However, the production of large single crystals
under hydrothermal conditions is very long and cumbersome. In fact, some experi-
ments have been carried out for 6 months. Demianets et al.
[123]
have studied the sol-
ubility of sodalite in aqueous solutions of NaOH (10
40 wt%) between 200
Cand
300
C.
Figure 6.25
shows the dependence of solubility of sodalite and the NaOH con-
centration at constant temperatures. The soluble curve of natural sodalite (hackmanite)
lies rather below the curve plotted for the solubility of artificial sodalite. The synthetic
mineral dissolves considerably at a rapid rate.