Geoscience Reference
In-Depth Information
Al
Si
Al
Si
Al
OH
-
Si Si
Al
Si
Al
Si
Si
Si
Al
Al
Hydrated species
Depolymeri-
zation
Al
Si
Al
Si
Si
Si
Al
Tetradron
Building block
Si
Al
Si
Al
Al
Si
Rearrangement around
hydrated species
Si
Al
Si
Al
Al
Si
Polyhedra
Si
Al
Al
Si
Si
Al
Further condensation
reaction
Si
Al
Si
Al
Si
Si
Al
Si
Al
Si
Al
Zeolite crystals
Growth of crystals
Initial
aluminosilicate gel
Amorphous
solid phase
Liquid phase
Hydrated species:
Na
+
, OH
-
, Al(OH)
-
,
(HO)
4-n
Si(O)
-
,
(AI, O, Si, OH)-Anions
0.1 < [Si] [Al]
×
10
2
< 6
(approximately)
OH
HO
OH
OH
OH
HO
OH
Heating
HO
Si
Al
Hydrated cations
1
≤
Si : Al
<
5
(O-Atoms not shown)
Crystallizing gel
Amorphous
solid phase
t*, dissolving
Increase of concentrations
in the liquid phase
Liquid phase
Condensation
reactions
Autocatalytic growth
of dissolution rate
of amorphous phase
Teme growth in size and
number of nuclei
Accumulation of
zeolite crystals
Formation of
nuclei
Decrease of concentrations
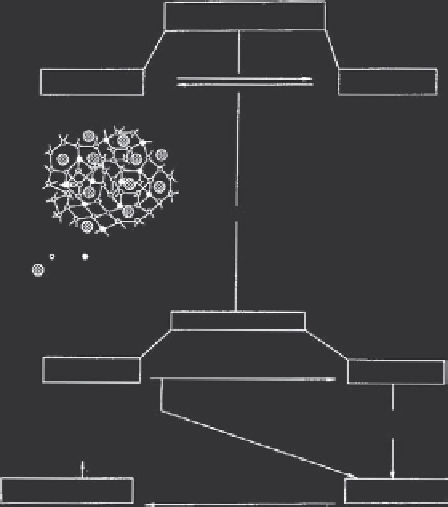
Figure 6.6 (a) Schematic illustrations of the solution-mediated transport. (b) Solid-hydrogen
transformation crystallization mechanism
[10]
.