Geoscience Reference
In-Depth Information
turn connect to form the infinitely extended frameworks of the various specific zeo-
lite crystal structures. Different combinations of the same SBU may give numerous
distinctive structural polyhedra formed from smaller ring units. The net negative
charge on the framework is balanced by the presence of cations, in most cases Ca,
Na, or K, which are situated in cavities within it. The zeolite framework is structur-
ally almost independent of the (Na, Ca, K) cations, and, as the latter do not fill all
the cavities, replacements of the type Ca
2(Na, K) can also occur.
Figures 6.1
and 6.2
show the basic structure of zeolite frameworks
[21,22]
. The structural com-
plexity of zeolites is because of the various ways in which the tetrahedral groups
are linked by the common sharing of oxygen ions to form polynuclear complexes.
Considerable variation in the chemical composition results from the substitution
of cations, such as those listed in
Table 6.4 [22]
. They give rise to zeolite and
zeolite-like materials of a very wide diversity. Thus, some simple criteria are used
to establish the zeolite frameworks. The most important one is the framework
density (FD), which means the number of temperature atoms (T-atoms) per
1000
˚
3
.
Figure 6.3a and b
shows the distribution of these values for porous and
dense frameworks whose structures are well established
[23]
.In
Figure 6.3a
,
2
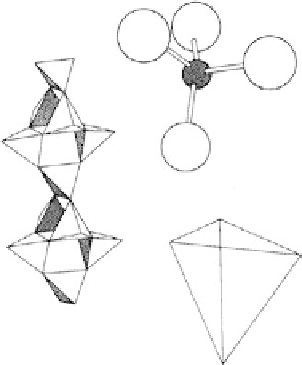
Figure 6.1 Basic structure of zeolite frameworks
[21]
.
Figure 6.2 Basic structure of zeolite frameworks
[22]
.
a
a