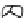Geoscience Reference
In-Depth Information
C
co
2
(mg/ml)
1000
600
500
300
400
0
350
300
200
14
12
13
10
9
250
11
8
100
7
6
200
5
4
3
2
1
50
100
0
100
200
300
400
T (
°
C)
Figure 5.46 Isobaric diagram for CO
2
[178]
.
hydrogen ions increases. The solubility of calcite in aqueous solutions of carbon
dioxide was studied between 10
300
C and 1
100 bar
[184,185]
. At the same
time, for T
constant,
the solubility rises exponentially with increasing P
CO2
.
5
600
C and 200
Later experiments at 200
100 mg/ml
CO
2
showed that absolute solubilities of calcite at high temperatures and pressures
were almost an order of magnitude lower than those measured at pressures up to
100 bar
[186]
. The following groups have carried out extensive studies on the solubil-
ity of calcite: (i) Ikornikova of Russia, during 1960s and 1970s—using chloride solu-
tions; (ii) Belt, the United States, during 1970s—using carbonate solutions;
(iii) Hirano, Japan, during 1980s and early 1990s—using nitrate solutions; (iv)
Kodaira, Japan, during 1990s—using H
2
O
1400 bar in solutions of 10
CO
2
; (v) Yamasaki, Japan, during 1990s
to date—using ammonium acetate and other organic solvents. Each one of the last
three groups claims the superiority of their solvents over the others.
Figures 5.47 and
5.48
show the solubility curves for calcite in different solvents. Yanagisawa et al.
(1996, 2001)
[182,187]
have studied extensively the effect of pH and increase of car-
bon number in alkyl group of the ammonium monocarboxylates solvents on the
growth of calcite and also the effect of dopant metals on the dislocation density of cal-
cite crystals. It was found that calcite crystals could not grow in the CH
3
COONH
4
solutions with a low pH. This has been explained by the change in the existing ion
species in the solution. A high growth rate was obtained in the solutions with a pH of
7.0
7.5. Beyond a pH of 7.5, the growth rate falls again. The pH of the solution