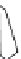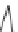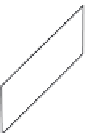Geoscience Reference
In-Depth Information
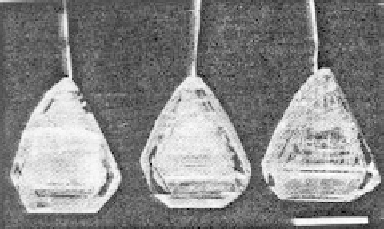
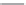
Figure 5.44 Characteristic photographs
of KTA single crystals obtained by Belt
and Ings under hydrothermal conditions
[158]
.
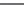
(001)
(201)
(011)
(100)
(111)
(110)
(201)
(011)
Figure 5.45 Ideal morphology of KTA crystals is shown schematically
[158]
.
compared to other KTP-like compounds, Nb-doped KTP crystals are observed to
be stable, and K
1
2
x
Ti
1
2
x
Nb
x
OPO
4
single crystals with x
0.25 have been
obtained without any charge compensator. Partial charge compensation of Nb
5
1
in
K
1
2
x
Ti
1
2
x
Nb
x
OPO
4
crystals by Ga
3
1
,Fe
3
1
, and Mg
2
1
has been reported.
However, complete substitution of Ti
4
1
in single crystals of KTP with any aliova-
lent cation pair has not been achieved by any method.
On the contrary, complete substitution of Ti
4
1
with Nb
5
1
Mg
2
1
and
Nb
5
1
Zn
2
1
cation pairs was reported to be possible in the case of KTA crystals.
This result by Chani et al. (1997)
[160]
suggested that it might be possible to
prepare new aliovalent analogous of KTA containing Nb
5
1
M
3
1
(M
Al, Cr, Ga,
Fe, In) cation pairs in octahedral positions of the KTP structure. KTA crystal was
the chosen basic material for this purpose as it was noted to be the most stable
compared to KTP
[159]
.
Lattice parameter values, in general, were found to increase with increasing
M
3
1
cation radius, as expected. The results of compaction measurements indicate
that Ti-free crystals with the structure of KTP have tendency to create potassium
vacancies. This result is in good agreement with that reported for nonsubstituted
KTP crystals. Structural analogues of KTP corresponding to K[Ta,M]O AsO
4
were
prepared by solid-state reaction. However, no KTP phase formation was observed
in K[Nb,M]O PO
4
and K[Ta,M]O PO
4
systems.
5