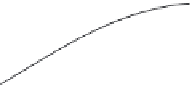Geoscience Reference
In-Depth Information
(prepared by sol
gel method) has been used as the nutrient with Ga:P ratio
1:1.5.
5
300
C,
The experiments were carried out
in the temperature range 200
5
C. The
yield of GaPO
4
single crystals as a function of growth temperature and concentra-
tion of different solvents used is shown in
Figures 5.29 and 5.30
. All the curves
shown in
Figures 5.29 and 5.30
are described by a simple exponential equation
M
P
20
35 MPa using Teflon liners, with a temperature gradient of 4
5
KC
n
, where K is the rate of bulk crystallization, C is the solvent concentration,
and n is the formal order of bulk crystallization.
Table 5.11
gives the values of K,
C, and n for aqueous HCl, HNO
3
, KF, and NaOH at 200
C, 250
C, and 300
C.
Also, the authors
[129]
have calculated the activation energy for the formation
of GaPO
4
single crystals from the Arrhenius equation. Similarly, the solubility of
α
5
-GaPO
4
has been studied by several workers within a wide range of PT conditions
(T
500
C, P
1000 atm)
[120,122,126,132]
.
Figure 5.31
shows the sol-
ubility of GaPO
4
in the phospho-sulfuric acid solution 15 M H
3
PO
4
1
200
50
5
5
9MH
2
SO
4
in terms of % in H
2
SO
4
. There is an increase in the solubility with a progressive
enrichment in H
2
SO
4
in the solvent. However, it always remains lower than in pure
H
2
SO
4
. The solubility remains retrograde in all the studies up to 400
C.
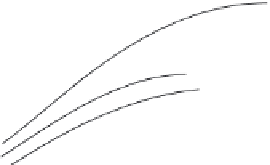
Figure 5.29 Yield of GaPO
4
single
crystals as a function of growth
temperature and concentration of
different solvents
[129]
.
M (g/day)
(a)
4
1.0
0.8
0.6
0.4
0.2
3
2
1
4
2.1
(b)
1.5
3
2
0.9
1
0.3
4
(c)
2.5
3
2
1.5
1
0.5
0.4
1.2
2.0
2.8
C
(M)