Geoscience Reference
In-Depth Information
5.4.1 Crystal Growth of Gallium Phosphate
The synthesis of gallium orthophosphate does not differ much from the synthesis of
berlinite. It is usually carried out by the hydrothermal technique at temperatures less
than 250
C. On the whole, the growth of aluminum and gallium phosphates is car-
ried out using reverse temperature gradient, or slow temperature, increasing essen-
tially in the acid media like H
3
PO
4
[128]
,H
2
SO
4
, HCl
H
2
SO
4
[121,126,127]
, HCl
[126,129]
, KF, NaOH
[129]
. The starting material/nutrient for
GaPO
4
growth is prepared usually by solid-state reactions with Ga
2
O
3
and
NH
4
H
2
PO
4
, twice at 1000
C for 24 h and subsequently hydrothermally treated at
150
C for 30 h in 4 M H
3
PO
4
solution. The resultant product is generally a single
phase of GaPO
4
. However, in recent years, the use of organic solvents and surfac-
tants has brought down the pressure and temperature conditions of synthesis. But the
size of the crystals is a major issue in solvothermal growth. Like berlinite, the gal-
lium phosphate also shows a retrograde solubility in all the solvents below 400
C,
and above this temperature, the solubility becomes positive
[118,122,129,130]
.
Hence, the nutrient is kept at the low-temperature zone (i.e., dissolution region), and
the seed crystal is kept at higher temperature zone (i.e., crystallization region). The
temperature gradient (5
H
2
SO
4
,H
3
PO
4
1
1
40
C) is selected according to the quality and the growth
rate required.
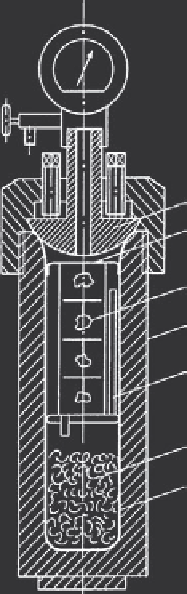
Figure 5.28
shows the schematic representation of the growth of
Figure 5.28 Schematic representation of the growth of berlinite
crystals
[128]
.
1
2
3
4
5
6
7