Geoscience Reference
In-Depth Information
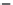
Figure 4.13 Stability diagrams,
speciation diagrams, and yield
diagrams
[82]
.
433 K
1
2
3
0
PbTiO
3(s)
+
Pb(OH)
2(s)
+
PbO
(s)
-2
PbTiO
3(s)
+
Pb(OH)
2(s)
Pb/Ti = 1.1
-4
Pb/Ti = 2.0
-6
PbTiO
3(s
)
Pb
2+
PbO
(aq)
-8
HPbO
2
-
PbOH
+
-10
0
2
4
6
8
10
12
14
pH
In the recent years, microwave hydrothermal is being popularly used to greatly
enhance the reaction kinetics. Also, efforts have been made to have better control
over the particle size, shape, yield, and purity
[86
88]
. However, the reaction
kinetics of most of the compounds under microwave hydrothermal have not been
understood precisely.
4.5.1 Experimental Investigations of Solubility
The selection of a suitable solvent and the theoretical aspects of solubility have
been discussed in the previous section. Here, let us consider the experimental
aspect, its determination, and role in growing single crystals. There are two princi-
pal methods for determining the solubility under hydrothermal conditions. The first
one is a sampling technique and has been described in detail by Morey and
Hesselgesser
[89]
. It is especially useful where two fluid phases are present. In this
technique, a suitably designed valve is arranged so that a fluid sample small
enough so as not to perturb equilibrium can be withdrawn and chilled from an iso-
thermal hydrothermal autoclave at operating conditions. If only one fluid phase is
present, a weight loss method has been proved to be somewhat less cumbersome
[90]
. It is quite difficult to study the solubility of crystals under hydrothermal con-
ditions if the compound does not form any metastable solutions over a wide tem-
perature range. If there is a considerable metastable range of this kind, however,
we may use the least troublesome method of studying solubility at high tempera-
tures and high pressures—the method of weight loss. If a weighed single crystal (or
crystals) is placed in the capsule under conditions where the phase is stable and