Geoscience Reference
In-Depth Information
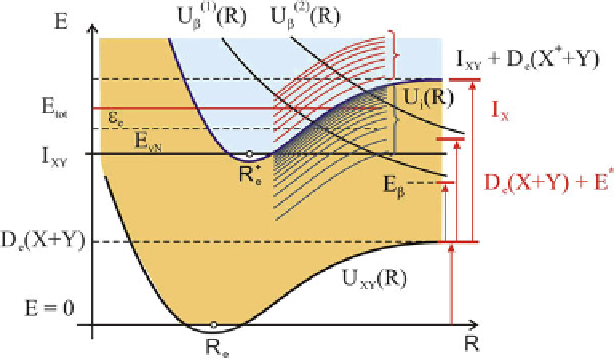
Fig. 1.6
Diabatic potential curves of the molecule XY for the case of I
X
<I
Y
.TermsU
.1
ˇ
.R/
and U
.2
ˇ
.R/ are responsible for the exothermic and endothermic AI reaction, respectively
vibrational motion, as it is necessary to account a broad range of the interatomic
coordinates, which is a separate independent task. The problem becomes even more
complicated when the bound states of the intermediate valence (non-Rydberg) X
*
Y
and ionic X
C
Y
configurations are included in the general scheme (Eqs.
1.13
,
1.15
,
1.21
,
1.22
,
1.23
).
1.4.3
Stochastic Regime of AI Reaction Diffusion Approach
Provided at I
X
<I
Y
, the heat of the AI reaction depends on the initial excitation
energy
E
*
of the X
*
atom. Indeed, when at a distance of R
!1
the energy position
E
C
D
e
.X
C
Y/>I
XY
is located over the ionization threshold of the XY molecule,
the AI reaction is exothermic (here
I
XY
and
D
e
(X
C
Y) are the ionization potential
and the dissociation energy of the XY molecule, respectively). In the opposite case,
the endothermic process with a reaction threshold of E
ˇ
D
I
XY
D
e
.X
C
Y/
E
takes place. Moreover, subject to
E
D
E
k
C
E
C
D
e
.X
C
Y/>I
XY
C
D
e
X
C
C
Y
(1.39)
(
E
,and
D
e
(X
C
C
Y) are the total and dissociation energies of the XY
C
ion), when
the total energy of the system exceeds the threshold for dissociation of the X
C
C
Y
ion channel, the exothermic reaction of the AI reaction should be suppressed
markedly. The presence of exact equality (Fig.
1.6
)
D
e
.X
C
Y/
C
I
X
D
I
XY
C
D
e
X
C
C
Y