Geoscience Reference
In-Depth Information
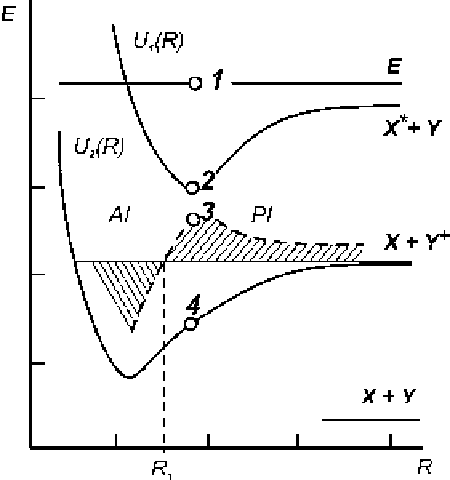
Fig. 1.1
Potential curves for
the molecular ion U
2
(
R
)and
the quasi-molecular system
U
1
(
R
). The value E
corresponds to the total
energy of the system. The
excitation energy of the atom
Y
*
is greater than the
ionization potential of the
atom X
possible. A diagram of the electron excited quasi-molecule XY
*
potential curves
and the ground state molecular ion XY
C
, qualitatively illustrating a separation
possibility of the processes
(
XY
C
C
e
X
C
C
Y
C
e
associative ionization.AI/
.1:8/
X
C
Y
!
n
type Penning AI reaction.PI/
.1:9/
(when the adiabatic approximation takes place), is shown in Fig.
1.1
. An implemen-
tation of the Franck-Condon principle in the conservation of the nuclei energy is
illustrated in this figure by presentation of the ordinate differences of points 1, 2
and 3, 4, respectively: U
1
.R/
U
2
.R/
D
U
3
.R/
U
4
.R/. When point 3 lies above
the dissociation ion XY
C
limit, the PI channel is preferred. If an autoionization
decay from the
U
1
(
R
) term for the interatomic distances
R
>
R
(1) takes place, the
PI-type reaction with formation of the atomic ion and one electron happens (reaction
(
1.3
)). Autoionization decay of
R
<
R
(1) leads to the formation of the molecular ion
in the stable vibrational excited state. In the same approximation, the energy of
electrons releazed in the reaction channels at the interatomic distance
R
is equal to
the
U
1
(
R
)
U
2
(
R
) difference. From analysis of the electron energy distribution in
the reactions (
1.2
and
1.3
), we can judge not only about the relative efficiency of the
molecular and atomic ion processes as the branching factor of the reaction, but also
about the behavior of potential curves of the electronically excited molecules. The
limiting value of the atoms (temperature) average energy, where the basic transition