Geoscience Reference
In-Depth Information
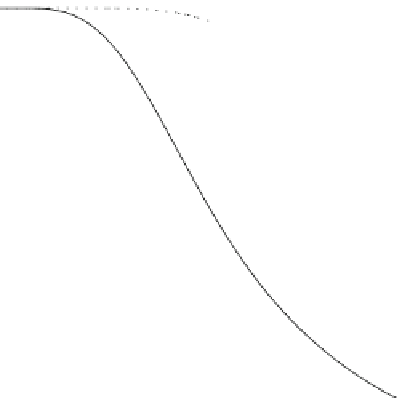
Fig. 3.20
Same as in
Fig.
3.19
, but for repulsive
potential. In contrast to
attraction, in this case the free
molecule limit works even
when the particle size is
comparable to the ion mean
free path
1.0
0.8
0.6
0.4
l
c
=1
l
c
=3
0.2
0.5
1.0
1.5
2.0
2.5
3.0
PARTICLE RADIUS
a
(in units of
l
)
where ˛
fm
.a/
D
˛
fm
.a;R
D1
/.
Let us analyze the expression for charging efficiency (Eq.
3.185
) in the free
molecule limit a
l. First, we notice that at a
l the denominator in Eq.
3.185
can be always replaced by unity. Then for the recombination rate Eq.
3.175
gives
˛.a/
a
2
v
T
Œ1
C
ˇ
j
U.a/
j C
ˇ
j
U.4D=
v
T
/
j
e
ˇjU
.
4D=
v
T
/
j
(3.187)
To obtain the widely cited free molecule limit ˛.a/
D
a
2
v
T
Œ1
C
ˇU.a/,the
term ˇU .4D=
v
T
/ should be small compared to 1. The inequality ˇU .4D=
v
T
/
1
does not hold for the Coulomb potential at ambient conditions. Indeed, l
c
D
ˇe
2
6
10
6
cm
l. For the attractive potential given by Eq.
3.1
one finds
1
C
qQ
l
c
a
1
C
e
qQl
c
v
T
=4D
:
q
2Q
"
1
"
C
2
C
qQ
l
c
v
T
4D
˛.a/
a
2
v
T
(3.188)
Figure
3.19
clearly demonstrates the role of the Coulomb distance in the case
of the Coulomb attraction. It is seen that even at small particle sizes a
l the