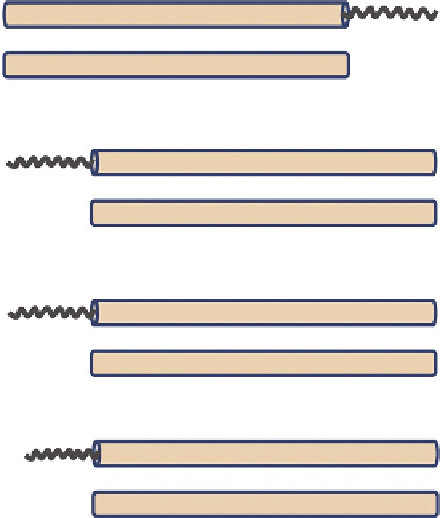
Biology Reference
In-Depth Information
1
389
C
NEIL1 (human)
N
263
1
Endo VIII (
E. coli
)
C
N
312
1
NTH1 (human)
C
N
211
1
Endo III (
E. coli
)
N
C
535
1
MYH (human)
C
N
1
350
MutY (
E. coli
)
N
C
318
1
C
APE1 (human)
N
268
1
Xth (
E. coli
)
N
C
F
IG
. 2. Disordered terminal extensions in human (and other mammalian) early BER/SSBR
proteins that are absent in their
E. coli
prototypes (
not drawn to scale)
. In many cases, disordered
segments were deleted for X-ray crystallographic structure analysis, which are consistent with
PONDR prediction.
8,90
The size range of disordered extensions in early BER proteins is 50-100
residues, with few exceptions, for example, hOGG1 and human Pol
b
, which
have short (
10 residues) disordered segments at both termini.
90
A. Functions of Disordered Terminal Extensions
The nonconserved, mostly disordered terminal peptide segments of early
BER/SSBR proteins in mammals implicate these in important functions in-
cluding damage sensing, protein-protein interactions, repair regulation via
posttranslational modifications, and nuclear localization signal (NLS).
8,90
Fur-
thermore, the disordered regions provide an opportunity for alternative splic-
ing without perturbing the structured regions.
95
Disorder also provides size
advantage in a polypeptide by providing a common interface for multiprotein
binding and sites of covalent modifications. Thus, disorder may help higher
organisms limit protein size and reduce intracellular crowding.
96