Environmental Engineering Reference
In-Depth Information
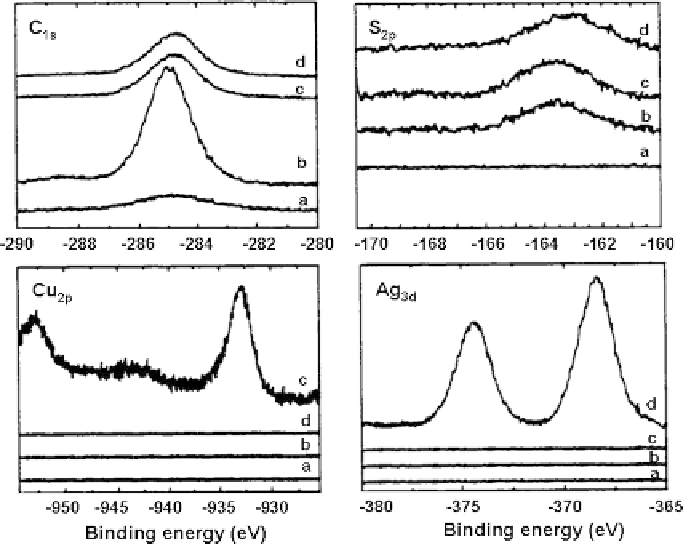
affinity to various metal ions such as gold, silver, and copper. This application is
illustrated in our loading tests of Cu
2+
and Ag
+
on MHA-coated g-Fe
2
O
3
particles from 10 mmol/L CuSO
4
or AgNO
3
solutions. The narrow-scan XPS
spectra of the loaded particles separated from the liquid/suspension with a hand
magnet are shown in Fig. 6.14. The load of Cu
2+
and Ag
+
on the MHA-coated
particles is evident from the presence of Cu
2p
(934 and 954 eV) and Ag
3d
(368
and 374 eV) XPS bands on spectra, respectively. The area ratio of the copper
satellite band to its 2p
3/2
band is lower than expected, suggesting that some of
the copper ions are in the cuprous state. It appears that some of cupric ions were
reduced to cuprous ions, accompanied by the oxidation of thiol to disulfide,
which accounts for the presence of an additional sulfur band of higher binding
energy but lower intensity. The surface metal-to-sulfur atomic ratio was found
to be 0.6 and 0.8 for copper and silver, respectively. These results suggest a 1:2
(metal-to-sulfur) binding for divalent copper and 1:1 binding for monovalent
silver. It is clear that the metal loading efficiency is sufficient for the MHA-
coated magnetic sorbents to be used in the removal or recovery of Ag
+
and
Cu
2+
from industrial effluents.
Fig. 6.14 XPS spectra of narrow scans for the interested elements (a) g-Fe
2
O
3
; (b) thiol-type
magnetic sorbents; (c) after copper loading; and (d) after silver loading