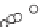Geoscience Reference
In-Depth Information
10
8
Minerals from
crustal rocks
(Rumble
et al
.)
6
4
Slope = 0.5
2
0
-2
-4
-6
-8
-10
-15
-10
-5
0
5
10
15
δ
18
O
Figure 3.7
17
Oand
18
Ofor
Relationship between
500 minerals from crustal rocks, mostly from China
(Rumble
et al.
, 2007). The values define a nearly perfect straight line (errors are smaller than
symbol size) going through (0,0) and with a slope of 0.5 demonstrating the perfect dependence
of oxygen isotope fractionation on the mass difference between masses at the numerator and
denominator. Laser ablation data by courtesy of Doug Rumble. Meteoric waters and sediments fall
on the same line, called the terrestrial fractionation line.
δ
δ
>
17
O:
Likewise for
δ
17
O
2
−
δ
17
O
1
≈
δ
1000
f
(3.32)
18
O, isotope fractionation makes the isotope compo-
sition of sample 2 move on a straight line going through sample 1 with a slope of
17
O and
y
In a plot with
x
=
δ
=
δ
1/2
(see below for details on this value). This pattern is actually quite general and, for a planet
starting with an isotopically homogeneous reservoir of oxygen, all the differentiation and
mixing processes produce samples with
≈
18
O plotting on this very same straight
(we just used an expansion of ln
17
O and
δ
δ
α
with respect to
m
), kinetic and biological processes
18
O values of essentially all terrestrial
samples plot on the terrestrial fractionation line (
Fig. 3.7
). It will be seen later that one very
important application of this simple theory is in the discrimination of planetary material.
The same principle holds for the isotopic variability of all the elements with more than two
stable isotopes, such as Fe, Zn, and Mo: if
17
O and
must also follow the same pattern. The
δ
δ
m
y
are the mass differences rela-
tive to the isotopic ratios used for
x
and
y
, respectively, the mass fractionation line has a
slope of
m
x
and
m
x
. Some rare outliers exist, due to photodissociation reactions (different
isotopes of the same element absorb radiations with slightly different wavelengths) or auto-
catalytic effects. Atmospheric sulfur with isotope ratios
34
S
m
y
/
32
S and ozone
are prime examples: these exotic processes are known by the name of mass-independent
32
S and
33
S
/
/