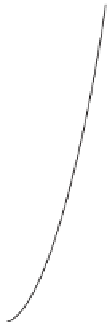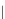Geoscience Reference
In-Depth Information
Harmonic oscillator approximation
hard bond (
k
2
)
soft bond (
k
1
)
1
k
=
2
M
molecule 1
E
n
molecule 2
Figure 3.5
Comparison between two bonds, one soft (left) and one hard (right) with the force constant of
the soft bond (
k
1
) less than that of the hard bond (
k
2
). The total energy is at a minimum when
heavy isotopes preferentially populate the hard bond.
This quantity is clearly
0 which indicates that, in order to achieve energy reduction, reac-
preferentially into the liquid phase. It is a general result that heavy isotopes fractionate in
favor of the stiffest bonds. Since transforming solid or liquid into vapor requires addition of
energy to break bonds (latent heat), solids and liquids, where most of the energy is stored in
vibrations, lower the total energy of the system by concentrating heavier isotopes. In con-
trast, vapor offers sites that are higher on the energy scale than the corresponding liquid
and therefore tend to concentrate the lighter isotopes relative to the liquid. In a liquid-
vapor equilibrium, such as H
2
O liquid and vapor below 220
◦
C, the liquid is enriched in
the heavier isotope (e.g.
18
O,
2
H), while the vapor preferentially concentrates the lighter
isotope (e.g.
16
O,
1
H).
>
The Boltzmann distribution
Let us consider
N
identical particles, atoms or molecules, which can occupy a large number of
energy levels,
n
1
being at the level
E
1
,
n
2
at the level
E
2
, etc. We assume that each individual
particle has an equal probability to land on any energy level. Without loss of generality, we
will assume that
N
is the Avogadro number (1 mole). We are going to justify to some extent
that the fractional proportions
f
i
=
n
i/N
of these atoms or molecules that have the energy
E
i
at temperature
T
is:
E
i
RT
e
−
f
i
=
const
×
(3.12)
Our assumptions are that the occupancy of a particular energy level, say
E
i
, is independent
of the occupancy of another level, say
E
j
. The function
f
therefore requires that
f
i
+
j
=
f
j
.
The only function with such a property is the exponential function, which suggests the form:
f
i
×
e
−
βE
i
f
i
=
const
×
(3.13)