Geoscience Reference
In-Depth Information
1 cm
pyroxene
corundum
garnet
amphibole
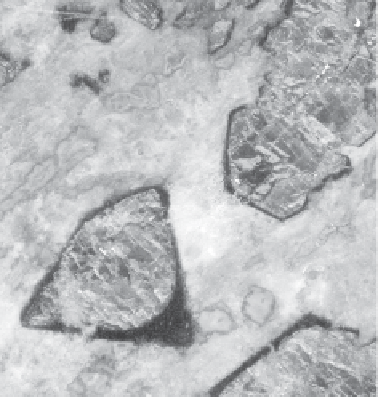
Figure 2.2
In this ancient metamorphosed basalt (eclogite), the fall in pressure when the rock rose to the
surface destabilized the mineral assemblage. Pyroxene and garnet reacted with fluids no longer
visible to give amphibole and corundum (sample courtesy of P. Thomas).
Representing compositions in vector form also sheds light on the concept of reaction
assemblage, or mineral reaction, that is so useful in metamorphic petrology.
Figure 2.2
shows a natural assemblage of minerals where pyroxene and garnet have reacted to produce
amphibole and corundum.
Let us illustrate these principles through a simpler mineral assemblage, i.e. the quartz
(SiO
2
), forsterite (Mg
2
SiO
4
), and enstatite (Mg
2
Si
2
O
6
) assemblage. A rock composed
of two components or, as is customary in petrology, two oxides, cannot have more than
two minerals co-existing at equilibrium. Three minerals cannot be stable simultaneously
because, in (SiO
2
, MgO) space, any “rock” vector could be represented by an infinite
number of combinations of “mineral” vectors whose equivalence is represented by the reac-
tion forsterite
enstatite (
Fig. 2.3
). Thermodynamics tells us that this reaction
evolves spontaneously from left to right, causing complete consumption of either quartz or
forsterite. The stable mineralogical assemblage therefore includes only two minerals: the
enstatite and, depending on the composition of the rock, either the quartz or the forsterite.
Geochemists are fond of ratios of elements or isotopes. There are two reasons for this:
ratios are generally measured more precisely than concentrations and they are less sensitive
to dilution phenomena. For example, the abundance of olivine in a basalt does not alter the
ratio of those elements that are segregated in the melt (incompatible elements). The evapo-
ration of seawater concentrates Cl
−
,Na
+
, ions, etc., but does not alter their concentration
ratios. It is a serious mistake to try to apply the rules for absolute concentrations to ratios.
The results stated below are explained in
Appendix B
.
For the ratio A/B (e.g. the Si/Al
ratio), the conservation equations become:
compos.
j
ϕ
+
quartz
⇔
B
j
=
1
(2.8)
C
A
C
B
C
A
C
B
B
j
0
=
ϕ
(2.9)
j
j