Geoscience Reference
In-Depth Information
S
M a x i m u m o f S
U
S
=
S (U, V)
M i n i m u m o f U
V
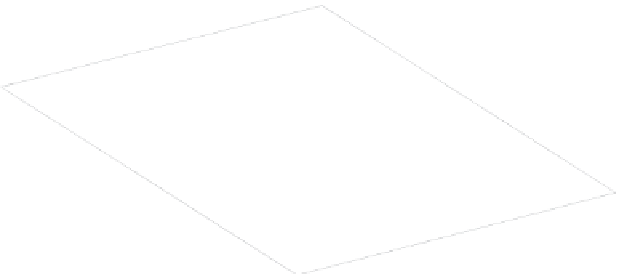
Figure C.1
The complementarity of the maximum entropy and minimum energy conditions are expressed by
the single equation
S
S(U,V)
representing the equation of a surface in (
U, S, V
)space.At
constant energy
U
(i.e. for an isolated system), the volume
V
of the system evolves
spontaneously so that its entropy is at a maximum. At constant entropy (no heat exchange with
the outside), the same system evolves toward a minimum energy state.
=
H
. For a system for
which the temperature
T
and pressure
P
are now prescribed, there is nothing to preclude
an energy exchange with the outside, whether thermal or mechanical. The intrinsic energy
of such a system is measured by another conservative magnitude, free enthalpy or Gibbs'
free energy
G
, so that:
U
, whereas in a calorimeter at constant pressure, this change is
G
=
U
+
PV
−
TS
(C.10)
Other forms of energy are used when control variables other than
T
and
P
are preferred.
Differentiating
G
and allowing for the definition of
U
gives:
d
G
=−
S
d
T
+
V
d
P
(C.11)
An apparently exotic property of
G
can be readily derived from the previous equation
and proves very useful for the study of chemical equilibria:
∂
(
G
/
T
)
P
=
H
(C.12)
∂
(
1
/
T
)
For a perfect gas, the equation of state reads:
PV
=
nRT
(C.13)
where
n
is the number of moles of gas in the enclosure and
R
is the gas constant. The
minimum energy (maximum entropy) corresponds to d
G
=
0. At constant temperature
and constant mole number, we can write:
nRT
d
P
P
d
G
=
V
d
P
=
(C.14)