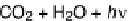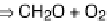Geoscience Reference
In-Depth Information
atmosphere
weathering
CO
2
CaAl
2
Si
2
O
8
+ 2
CO
2
+ 3H
2
O
⇒
Ca
2+
+ 2HCO
3
+ Al
2
Si
2
O
5
(OH)
4
CO
2
O
2
photosynthesis
ocean
C + O
2
⇒
CO
2
reduced carbon
(redox) cycle
oxidized carbon
(alkalinity) cycle
CO
2
sedimentation
C
CaCO
3
crust
volcanism
mantle
subduction
Figure 13.1
The long-term carbon cycles. The oxidized carbon cycle involves the production of alkalinity by
weathering of silicates and its consumption by carbonate precipitation (carbonate weathering
corresponds to recycling). The reaction shown on the right corresponds to the weathering of calcic
plagioclase into dissolved ions and kaolinite. The modern residence time of alkalinity in the ocean
is about 100 ka. Most of the reduced carbon is produced by photosynthetic plants which break
atmospheric and dissolved CO
2
into reduced carbon and oxygen. Respiration returns some of the
reduced carbon to carbon dioxide. The residence time of reduced carbon in the ocean is much
shorter than the residence time of oxidized carbon. Both reduced and oxidized carbon are lost to
the mantle by subduction of sediments, which leaves an excess of atmospheric oxygen.
Atmospheric carbon dioxide is replenished by volcanism.
The geochemical cycle of carbon is of particular significance because it is the most
essential component of life. The extremely diverse polymerization modes of carbon com-
pounds and their easy binding to a number of other elements and molecules (nitrogen,
phosphate, iron, magnesium, and scores of other metals) are unique in nature. A major
source of carbon dioxide is volcanic outgassing from the mantle. Carbonate groups are
essentially indestructible except by biological activity or in extremely reducing environ-
ments. The prime sites for production of reduced carbon from atmospheric CO
2
and
oceanic carbonates are the ocean surface, continental shelves, and continental biosphere.
This production, fuelled by photosynthetic processes, is called primary productivity.
Igneous and biogenic reduced carbon is easily oxidized by atmospheric oxygen during
weathering. The resulting carbon dioxide is distributed almost equally between atmo-
spheric CO
2
and oceanic carbonates. Burial and subduction of sediments rich in organic
carbon (reverse weathering) leaves unbalanced oxygen that accounts for the high propor-
tion of this gas in the atmosphere.
Figure 13.1
shows a simplified picture of the long-term
oxidized and reduced carbon cycles.