Geoscience Reference
In-Depth Information
2000
Refractory
minerals
Olivine
1500
Iron
Nickel
Feldspar
1000
FeS
FeO
Amphibole
500
Serpentine
Water
−
8
−
6
−
4
−
2
0
log
P
(bar)
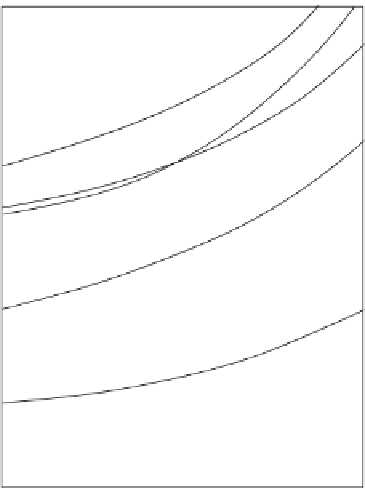
Figure 12.8
Condensation sequence of different minerals from the solar nebula calculated by adiabatic cooling
(without heat exchange) of a gas of solar composition (after Lewis,
1995
).
chondrites, falls to 10 000 for the Earth (probably 7000 for Mars), and to 3000 for the
Moon. This is consistent with the dramatic difference in
87
Sr/
86
Sr between chondrites on
the one hand, and the Earth and Moon on the other hand; Rb is an alkali element similar
to K, very volatile, whereas the alkaline-earth element Sr is quite refractory. Although the
distribution of Sr isotopes in chondrites has been disturbed by the low-temperature alter-
ation of their parent body, it is clear, as pointed out by Paul Gast in 1960, that the
87
Sr/
86
Sr
ratio of the Earth (
0.709) is much less radiogenic than the mean ratio of chondrites or
of the solar photosphere (
≈
0.745), which attests to a very low Rb/Sr ratio for our planet
(
Fig. 12.9
). It is incontrovertible that the Earth, in addition to K, lost most of its Rb very
early after accretion. The Earth and the Moon even more are therefore particularly depleted
in volatile elements.
It is commonly said that the amount of water held by the terrestrial mantle (
≈
≈
200 ppm)
is similar to the mass of the ocean. An old idea pervading literature and based on the
analysis of basalts is that water from the oceans and most atmospheric gases were out-
gassed from the mantle throughout geological time. But if such a large proportion was lost
of elements (K, Rb) that only vaporize at temperatures in excess of 1000 K, how much
gas and water, with a much lower condensation point, could have been preserved in the
accreting material? Probably not much, so the Earth accreted essentially dry and water was
added later from a different source. This is a conundrum that we will address in the next
section.