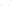Geoscience Reference
In-Depth Information
9.0
Fe
8.8
8.6
8.4
8.2
8.0
Fusion
7.8
Fission
7.6
7.4
0
50
100
150
200
250
Mass number,
A
Figure 12.2
Binding energy per nucleon of different nuclides is maximum for iron (in millions of
electron-volts). Fission of nuclides heavier than iron and fusion of nuclides lighter than iron are
energy-producing reactions and, apart from their activation energy, occur spontaneously.
Nucleosynthesis beyond iron will therefore consume energy.
highly energetic radiation produced in the stellar interior is therefore absorbed and further
converted into thermal energy: it can only escape at a wavelength corresponding to the
surface temperature of the star. The surface temperature of the Sun (6000 K) corresponds
to radiation in the window of visible wavelength.
Understanding these processes involves the concept of nucleon (protons and neutrons)
bonding energy. In the fission of a uranium atom the total mass of the fragments, e.g. xenon
and barium, is less than that of the initial atom. Conversely, in hydrogen atom fusion the
mass of the resulting atom, e.g. lithium, is less than that of the initial hydrogen atoms.
The extra mass is converted into thermal energy. Any reaction that releases energy is
liable to occur spontaneously. If we plot the binding energy thus available per nucleon
(
Fig. 12.2
) it can be seen that the maximum of the curve is located at the position of iron:
if the activation barriers are crossed the fusion reactions of light elements and fission reac-
tions of heavy elements will be spontaneous, while masses in the vicinity of iron will be
stable.
Let us now examine the abundance distribution of elements in the Sun relative to their
atomic number. A first observation is that elements with even atomic numbers are more
abundant than those with odd atomic numbers, reflecting the greater stability of nuclei
in which the nucleons are paired. This characteristic is one justification of element plots
against a reference standard such as chondrites, which overcomes the odd-even effect.
An irregular decline in abundance is observed with increasing mass number. In addition,
abundance peaks occur at certain mass numbers such as that of iron (56), or for other
nuclides with proton or neutron magic numbers of 2, 8, 20, 28, 50, 82, and 126. There is
also a considerable apparent deficit in the low-mass elements Li, Be, and B.
From hydrogen to iron, nuclear fusion within stars is the predominant process. The
fusion of hydrogen into helium is the dominant “low” temperature (10
7
-10
8
K) process