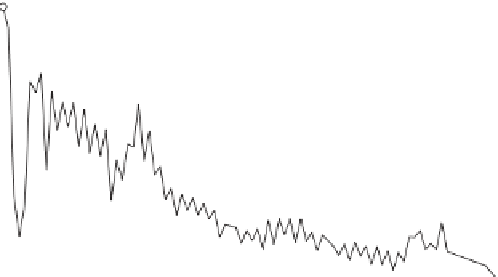Geoscience Reference
In-Depth Information
radioactive decay. Who would think that our warm sunshine is actually the output of the
largest nuclear reactor for light years around?
12.1 The formation of elements
The most abundant element in the universe is hydrogen, an element formed by an elec-
tron orbiting around a single proton. Let us begin with the formation of a Sun-like star
by gravitational collapse of an interstellar nebula, a cloud of gas and dust. The Sun itself,
which makes up 99.9% of the total mass of the Solar System, consists of 71% H, 27% He,
and 2% of heavier elements. The composition of the Solar System is given by spectro-
The accumulation of potential energy released by the collapse of the star's enormous mass
raises the elements at its center to extremely high temperatures, typically several million
K, until thermal agitation prevents further contraction. At these very high temperatures,
the elements are totally stripped of their electrons, which leaves the Sun largely made of
a dense gas of protons, electrons, and alpha particles. With the help of a tunnel effect,
opposite to that referred to for the
α
decay process, the nuclei gain enough thermal energy
to overcome the Coulomb repulsion barrier between them. The nuclei then fuse together
and thermonuclear reactions begin, releasing gigantic energy reserves by conversion of
mass. The star achieves steady state when the energy produced by nuclear reactions is
balanced by the emission of neutrinos generated in the nuclear reactions and by electro-
magnetic radiation in outer space. At high pressure, however, the stellar gas is opaque. The
10
+11
10
+9
O
10
+7
Fe
Neutron capture
10
+5
10
+3
Pb
10
+1
Be
10
-1
10
-3
0 0 0 0 0 0 0 0 0 0 0
Atomic number,
Z
Figure 12.1
Chemical composition of the Solar System standardized to a million atoms of Si. The main
nucleosynthetic stages are marked above the curve (after Pagel,
1997
). Notice the stability peaks
(e.g. Fe, Pb) and the lower abundance of elements with odd atomic numbers compared with their
even-numbered neighbors, whose nuclei are more stable. The deficit in the light atoms Li, Be, and
B results from destruction of these elements in the stellar interiors.